200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 6456-74-2
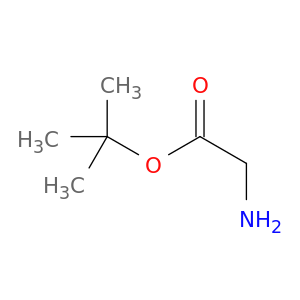
6456-74-2 | tert-butyl glycinate
CAS No: 6456-74-2 Catalog No: AG0038KC MDL No:MFCD00038194
Product Description
Catalog Number:
AG0038KC
Chemical Name:
tert-butyl glycinate
CAS Number:
6456-74-2
Molecular Formula:
C6H13NO2
Molecular Weight:
131.1729
MDL Number:
MFCD00038194
IUPAC Name:
tert-butyl 2-aminoacetate
InChI:
InChI=1S/C6H13NO2/c1-6(2,3)9-5(8)4-7/h4,7H2,1-3H3
InChI Key:
SJMDMGHPMLKLHQ-UHFFFAOYSA-N
SMILES:
NCC(=O)OC(C)(C)C
Properties
Complexity:
104
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
131.095g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
131.175g/mol
Monoisotopic Mass:
131.095g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
52.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.2
Literature
| Title | Journal |
|---|---|
| A powerful synergistic effect for highly efficient diastereo- and enantioselective phase-transfer catalyzed conjugate additions. | Chemical communications (Cambridge, England) 20110207 |
| Synthesis and structure-activity relationships of potent 1-(2-substituted-aminoacetyl)-4-fluoro-2-cyanopyrrolidine dipeptidyl peptidase IV inhibitors. | Chemical & pharmaceutical bulletin 20080801 |
| alpha,alpha'-disubstituted amino acids with silylated side chains as lipophilic building blocks for the synthesis of peptaibol analogues. | Chemistry & biodiversity 20080701 |
| Synthesis and ex vivo profiling of chemically modified cytomegalovirus CMVpp65 epitopes. | Journal of peptide science : an official publication of the European Peptide Society 20080301 |
| Glycine- and sarcosine-based models of vanadate-dependent haloperoxidases in sulfoxygenation reactions. | Inorganic chemistry 20070108 |
| Catalytic enantioselective synthesis of glutamic acid derivatives via tandem conjugate addition-elimination of activated allylic acetates under chiral PTC conditions. | Journal of the American Chemical Society 20051005 |
| Cinchona alkaloid-based polymer-bound phase-transfer catalysts: efficient enantioselective alkylation of benzophenone imine of glycine esters. | Molecular diversity 20050101 |
| A glycine-dependent riboswitch that uses cooperative binding to control gene expression. | Science (New York, N.Y.) 20041008 |
| Chemo-enzymatic synthesis of N-arachidonoyl glycine. | Biotechnology letters 20040801 |
| Acyclic stereoselective boron alkylation reactions for the asymmetric synthesis of beta-substituted alpha-amino acid derivatives. | Journal of the American Chemical Society 20030305 |
| The enantioselective synthesis of alpha-amino acid derivatives via organoboranes. | Journal of the American Chemical Society 20020814 |
| Chiral phosphine-free Pd-mediated asymmetric allylation of prochiral enolate with a chiral phase-transfer catalyst. | Organic letters 20011018 |
| Enantioselective synthesis of (R)-3-(3,4-dihydroxyphenyl)alanine from tert-butyl glycinate. | The Journal of organic chemistry 20010518 |
Related Products
© 2019 Angene International Limited. All rights Reserved.