200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 637-53-6
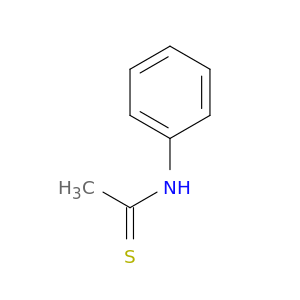
637-53-6 | N-Phenylethanethioamide
CAS No: 637-53-6 Catalog No: AG003US6 MDL No:MFCD00004942
Product Description
Catalog Number:
AG003US6
Chemical Name:
N-Phenylethanethioamide
CAS Number:
637-53-6
Molecular Formula:
C8H9NS
Molecular Weight:
151.2288
MDL Number:
MFCD00004942
IUPAC Name:
N-phenylethanethioamide
InChI:
InChI=1S/C8H9NS/c1-7(10)9-8-5-3-2-4-6-8/h2-6H,1H3,(H,9,10)
InChI Key:
MWCGLTCRJJFXKR-UHFFFAOYSA-N
SMILES:
CC(=S)Nc1ccccc1
EC Number:
211-288-4
UNII:
L9AL2GO03Y
NSC Number:
36984
Properties
Complexity:
116
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
151.046g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
151.227g/mol
Monoisotopic Mass:
151.046g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
44.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Arylazolyl(azinyl)thioacetanilides. Part 10: design, synthesis and biological evaluation of novel substituted imidazopyridinylthioacetanilides as potent HIV-1 inhibitors. | Bioorganic & medicinal chemistry 20120915 |
| 1,2,3-thiadiazole thioacetanilides. Part 2: Synthesis and biological evaluation of a new series of 2-{[4-(3,4-dichlorophenyl)-1,2,3-thiadiazol-5-yl]sulfanyl}acetanilides as HIV-1 inhibitors. | Chemistry & biodiversity 20100701 |
| 1,2,3-Selenadiazole thioacetanilides: synthesis and anti-HIV activity evaluation. | Bioorganic & medicinal chemistry 20090901 |
| Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry 20090815 |
| 1,2,3-Thiadiazole thioacetanilides as a novel class of potent HIV-1 non-nucleoside reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry letters 20081015 |
| Thioacetanilide at 120 K. | Acta crystallographica. Section C, Crystal structure communications 20080801 |
| Application of beta-(2-chloroaroyl) thioacetanilides in synthesis: an unusual and highly efficient access to thiochromeno[2,3-b]pyridine derivatives. | The Journal of organic chemistry 20080307 |
| Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. | Bioorganic & medicinal chemistry 20070601 |
| Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. | Bioorganic & medicinal chemistry 20060601 |
| Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant. | Bioorganic & medicinal chemistry letters 20060515 |
| Optical analysis of the cirrhotic liver by near-infrared time-resolved spectroscopy. | Surgery today 20040101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.