200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 6351-10-6
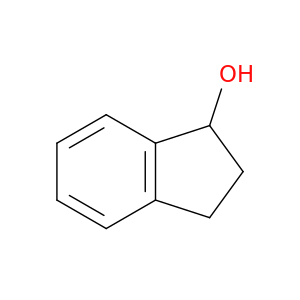
6351-10-6 | 2,3-Dihydro-1H-inden-1-ol
CAS No: 6351-10-6 Catalog No: AG003EF8 MDL No:MFCD00003797
Product Description
Catalog Number:
AG003EF8
Chemical Name:
2,3-Dihydro-1H-inden-1-ol
CAS Number:
6351-10-6
Molecular Formula:
C9H10O
Molecular Weight:
134.1751
MDL Number:
MFCD00003797
IUPAC Name:
2,3-dihydro-1H-inden-1-ol
InChI:
InChI=1S/C9H10O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-4,9-10H,5-6H2
InChI Key:
YIAPLDFPUUJILH-UHFFFAOYSA-N
SMILES:
OC1CCc2c1cccc2
NSC Number:
31258
Properties
Complexity:
122
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
134.073g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
134.178g/mol
Monoisotopic Mass:
134.073g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
20.2A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
1.5
Literature
| Title | Journal |
|---|---|
| Synthesis and biological evaluation of novel homochiral carbocyclic nucleosides from 1-amino-2-indanols. | Bioorganic & medicinal chemistry 20121001 |
| Synthesis, X-ray analysis, and biological evaluation of a new class of stereopure lactam-based HIV-1 protease inhibitors. | Journal of medicinal chemistry 20120322 |
| Gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices as new and efficient catalysts for alcohol oxidations. | Chemistry (Weinheim an der Bergstrasse, Germany) 20120109 |
| Structural features determining the intestinal epithelial permeability and efflux of novel HIV-1 protease inhibitors. | Journal of pharmaceutical sciences 20110901 |
| Efficient catalytic cycloalkane oxidation employing a 'helmet' phthalocyaninato iron(III) complex. | Dalton transactions (Cambridge, England : 2003) 20110614 |
| Synthesis and evaluation of indatraline-based inhibitors for trypanothione reductase. | ChemMedChem 20110207 |
| Rhodium(I)-catalyzed 1,4-silicon shift of unactivated silanes from aryl to alkyl: enantioselective synthesis of indanol derivatives. | Angewandte Chemie (International ed. in English) 20101227 |
| Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. | The Journal of pharmacology and experimental therapeutics 20101201 |
| Characterization of a rat NADPH-dependent aldo-keto reductase (AKR1B13) induced by oxidative stress. | Chemico-biological interactions 20090316 |
| Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. | Chemico-biological interactions 20090316 |
| Fluorescence spectroscopy of jet-cooled chiral (+/-)-indan-1-ol and its cluster with (+/-)-methyl- and ethyl-lactate. | The Journal of chemical physics 20061107 |
| An expedited approach to the vitamin D trans-hydrindane building block from the Hajos dione. | Organic letters 20060608 |
| Conformational landscapes and free-jet rotational spectrum of indan-1-ol. | Chemphyschem : a European journal of chemical physics and physical chemistry 20060313 |
| Electronic effects of ring substituents on triplet benzylic biradicals. | Organic letters 20060216 |
| Enzymatic properties of a member (AKR1C19) of the aldo-keto reductase family. | Biological & pharmaceutical bulletin 20050601 |
| Evaluation of (+)-sparteine-like diamines for asymmetric synthesis. | The Journal of organic chemistry 20040820 |
| Unexpectedly small ortho-oxygen substituent effects on stabilities of benzylic carbocations. | Journal of the American Chemical Society 20040818 |
| Degradation of aromatic hydrocarbons by Sphingomonas paucimobilis strain EPA505. | Archives of environmental contamination and toxicology 20040801 |
| Heterogeneous adsorption of 1-indanol on cellulose tribenzoate and adsorption energy distribution of the two enantiomers. | Analytical chemistry 20040101 |
| Modeling of the separation of two enantiomers using a microbore column. | Journal of chromatography. A 20031212 |
| Comparison of the binary equilibrium isotherms of the 1-indanol enantiomers on three high-performance liquid chromatography columns of different sizes. | Journal of chromatography. A 20031010 |
| Oxidative kinetic resolution of racemic alcohols catalyzed by chiral ferrocenyloxazolinylphosphine-ruthenium complexes. | The Journal of organic chemistry 20030725 |
| Determination of the single component and competitive adsorption isotherms of the 1-indanol enantiomers by the inverse method. | Journal of chromatography. A 20030711 |
| Equivalent models of indanol isomers adsorption on cellulose tribenzoate. | Biotechnology progress 20030101 |
| Improvement in the selectivity and metabolic stability of the serotonin 5-HT(1A) ligand, S 15535: a series of cis- and trans-2-(arylcycloalkylamine) 1-indanols. | Journal of medicinal chemistry 20020103 |
| Synthesis of enantiopure oxorhenium(V) and arylimidorhenium(V) '3 + 2' Schiff base complexes. X-ray diffraction, cyclic voltammetry, UV-vis, and circular dichroism characterizations. | Inorganic chemistry 20011217 |
| Enantioselective routes to both enantiomers of aryl alcohols with a single catalyst antipode: Ru and Os transfer hydrogenation catalysts. | Organic letters 20011115 |
| Expression and kinetic properties of a recombinant 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase isoenzyme of human liver. | Journal of biochemistry 19950801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.