200,000+ products from a single source!
sales@angenechem.com
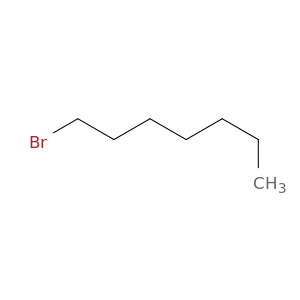
629-04-9 | 1-Bromoheptane
CAS No: 629-04-9 Catalog No: AG0032Q6 MDL No:MFCD00000273
Product Description
Catalog Number:
AG0032Q6
Chemical Name:
1-Bromoheptane
CAS Number:
629-04-9
Molecular Formula:
C7H15Br
Molecular Weight:
179.0980
MDL Number:
MFCD00000273
IUPAC Name:
1-bromoheptane
InChI:
InChI=1S/C7H15Br/c1-2-3-4-5-6-7-8/h2-7H2,1H3
InChI Key:
LSXKDWGTSHCFPP-UHFFFAOYSA-N
SMILES:
CCCCCCCBr
EC Number:
211-068-8
NSC Number:
7315
Properties
Complexity:
35.4
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
178.036g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
179.101g/mol
Monoisotopic Mass:
178.036g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
4.4
Literature
| Title | Journal |
|---|---|
| Microwave-assisted preparation of the quorum-sensing molecule 2-heptyl-3-hydroxy-4(1H)-quinolone and structurally related analogs. | Nature protocols 20120524 |
| Preclinical genotoxicology of nor-β-lapachone in human cultured lymphocytes and Chinese hamster lung fibroblasts. | Chemical research in toxicology 20110919 |
| 3,3'-[1,2-Phenyl-enebis(methyl-ene)]bis-(1-heptyl-benzimidazolium) dibromide monohydrate. | Acta crystallographica. Section E, Structure reports online 20110701 |
| Synthesis and evaluation of quinonoid compounds against tumor cell lines. | European journal of medicinal chemistry 20110101 |
| Synthesis of truncated analogues of the iNKT cell agonist, α-galactosyl ceramide (KRN7000), and their biological evaluation. | Bioorganic & medicinal chemistry 20110101 |
| Ontogeny and season constrain the production of herbivore-inducible plant volatiles in the field. | Journal of chemical ecology 20101201 |
| Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines. | Chemico-biological interactions 20101006 |
| 1,4-Di-n-hept-yloxy-2,5-dinitro-benzene. | Acta crystallographica. Section E, Structure reports online 20100101 |
| Biochemical mechanism of acetaminophen (APAP) induced toxicity in melanoma cell lines. | Journal of pharmaceutical sciences 20090401 |
| Biochemical mechanism of acetylsalicylic acid (Aspirin) selective toxicity toward melanoma cell lines. | Melanoma research 20081201 |
| Metabolic bioactivation and toxicity of ethyl 4-hydroxybenzoate in human SK-MEL-28 melanoma cells. | Journal of pharmaceutical sciences 20080501 |
| Biochemical basis of 4-hydroxyanisole induced cell toxicity towards B16-F0 melanoma cells. | Cancer letters 20061118 |
| The biosynthesis of ascorbate protects isolated rat hepatocytes from cumene hydroperoxide-mediated oxidative stress. | Free radical biology & medicine 20050401 |
| Glycogenolysis is directed towards ascorbate synthesis by glutathione conjugation. | Biochemical and biophysical research communications 20040423 |
| Glutathione depletion exacerbates methylenedianiline toxicity to biliary epithelial cells and hepatocytes in rats. | Toxicological sciences : an official journal of the Society of Toxicology 20030801 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.