200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 625-50-3
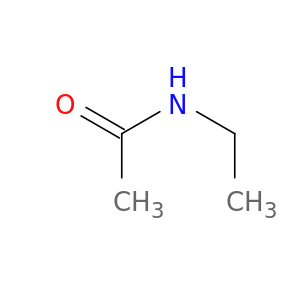
625-50-3 | N-Ethylacetamide
CAS No: 625-50-3 Catalog No: AG003T01 MDL No:MFCD00009029
Product Description
Catalog Number:
AG003T01
Chemical Name:
N-Ethylacetamide
CAS Number:
625-50-3
Molecular Formula:
C4H9NO
Molecular Weight:
87.1204
MDL Number:
MFCD00009029
IUPAC Name:
N-ethylacetamide
InChI:
InChI=1S/C4H9NO/c1-3-5-4(2)6/h3H2,1-2H3,(H,5,6)
InChI Key:
PMDCZENCAXMSOU-UHFFFAOYSA-N
SMILES:
CCNC(=O)C
EC Number:
210-896-7
UNII:
M9Y95ZE6J9
NSC Number:
406307
Properties
Complexity:
51.5
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
87.068g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
87.122g/mol
Monoisotopic Mass:
87.068g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
29.1A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.1
Literature
| Title | Journal |
|---|---|
| Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction. | Phytochemistry 20110801 |
| Fractional Stokes-Einstein-Debye relation and orientational entropy effects in strongly hydrogen-bonded liquid amides. | Physical chemistry chemical physics : PCCP 20110307 |
| Involvement of basal protein kinase C and extracellular signal-regulated kinase 1/2 activities in constitutive internalization of AMPA receptors in cerebellar Purkinje cells. | The Journal of neuroscience : the official journal of the Society for Neuroscience 20060503 |
| Synthesis, antioxidant activity and structure-activity relationships for a new series of 2-(N-acylaminoethyl)indoles with melatonin-like cytoprotective activity. | Journal of pineal research 20060401 |
| Identification of dielectric and structural relaxations in glass-forming secondary amides. | The Journal of chemical physics 20050801 |
| Substituent effects on the in situ activation of the double activated cross-linking reaction. | Nucleic acids symposium series (2004) 20050101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.