200,000+ products from a single source!
sales@angenechem.com
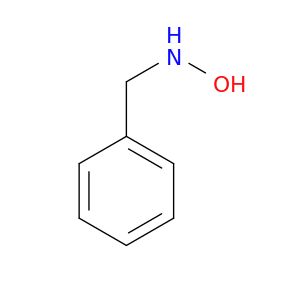
622-30-0 | N-Benzylhydroxylamine
CAS No: 622-30-0 Catalog No: AG0038LC MDL No:MFCD06654467
Product Description
Catalog Number:
AG0038LC
Chemical Name:
N-Benzylhydroxylamine
CAS Number:
622-30-0
Molecular Formula:
C7H9NO
Molecular Weight:
123.1525
MDL Number:
MFCD06654467
IUPAC Name:
N-benzylhydroxylamine
InChI:
InChI=1S/C7H9NO/c9-8-6-7-4-2-1-3-5-7/h1-5,8-9H,6H2
InChI Key:
LVCDXCQFSONNDO-UHFFFAOYSA-N
SMILES:
ONCc1ccccc1
Properties
Complexity:
69.3
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
123.068g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
123.155g/mol
Monoisotopic Mass:
123.068g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
32.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| Catalysis through temporary intramolecularity: mechanistic investigations on aldehyde-catalyzed Cope-type hydroamination lead to the discovery of a more efficient tethering catalyst. | Journal of the American Chemical Society 20121010 |
| NBHA reduces acrolein-induced changes in ARPE-19 cells: possible involvement of TGFβ. | Current eye research 20110401 |
| 3-[(E)-1-(Benzyl-oxyimino)eth-yl]-2-oxo-2H-chromen-7-yl acetate. | Acta crystallographica. Section E, Structure reports online 20100301 |
| Access to alpha-substituted amino acid derivatives via 1,3-dipolar cycloaddition of alpha-amino ester derived nitrones. | The Journal of organic chemistry 20100205 |
| Acrolein toxicity: Comparison with reactive oxygen species. | Biochemical and biophysical research communications 20090109 |
| N-benzyl aspartate nitrones: unprecedented single-step synthesis and [3 + 2] cycloaddition reactions with alkenes. | Organic letters 20081016 |
| A practical synthesis of (3R,4R)-N-tert-butoxycarbonyl-4-hydroxymethylpyrrolidin-3-ol. | Organic & biomolecular chemistry 20070907 |
| Highly efficient three-component synthesis of beta-lactams from N-methylhydroxylamine, aldehydes, and phenylacetylene. | Chemistry, an Asian journal 20060717 |
| Neurotoxicity of reactive aldehydes: the concept of 'aldehyde load' as demonstrated by neuroprotection with hydroxylamines. | Brain research 20060620 |
| Cyclopropylamine inactivation of cytochromes P450: role of metabolic intermediate complexes. | Archives of biochemistry and biophysics 20050415 |
| Enantioselective synthesis of alpha,beta-disubstituted-beta-amino acids. | Journal of the American Chemical Society 20031001 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.