200,000+ products from a single source!
sales@angenechem.com
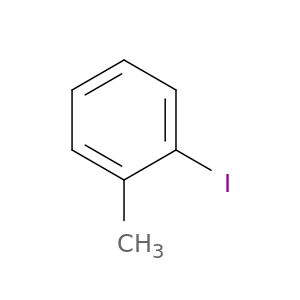
615-37-2 | 1-Iodo-2-methylbenzene
CAS No: 615-37-2 Catalog No: AG003HH1 MDL No:MFCD00001042
Product Description
Catalog Number:
AG003HH1
Chemical Name:
1-Iodo-2-methylbenzene
CAS Number:
615-37-2
Molecular Formula:
C7H7I
Molecular Weight:
218.0350
MDL Number:
MFCD00001042
IUPAC Name:
1-iodo-2-methylbenzene
InChI:
InChI=1S/C7H7I/c1-6-4-2-3-5-7(6)8/h2-5H,1H3
InChI Key:
RINOYHWVBUKAQE-UHFFFAOYSA-N
SMILES:
Cc1ccccc1I
EC Number:
210-422-9
UNII:
8OK4H85T07
NSC Number:
3774
Properties
Complexity:
70.8
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
217.959g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
218.037g/mol
Monoisotopic Mass:
217.959g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.9
Literature
| Title | Journal |
|---|---|
| Mizoroki-heck-type reaction mediated by potassium tert-butoxide. | Angewandte Chemie (International ed. in English) 20110509 |
| Spin-orbit ab initio investigation of photolysis of o-, m-, and p-iodotoluene. | The Journal of chemical physics 20100107 |
| Structures, biological activities, and total syntheses of 13-hydroxy- and 13-acetoxy-14-nordehydrocacalohastine, novel modified furanoeremophilane-type sesquiterpenes from Trichilia cuneata. | Organic letters 20050428 |
| Characterization of the electrophile binding site and substrate binding mode of the 26-kDa glutathione S-transferase from Schistosoma japonicum. | Proteins 20030401 |
| Synthesis of seven-membered lactones via nickel- and zinc-catalyzed highly regio- and stereoselective cyclization of 2-iodobenzyl alcohols with propiolates. | Journal of the American Chemical Society 20020522 |
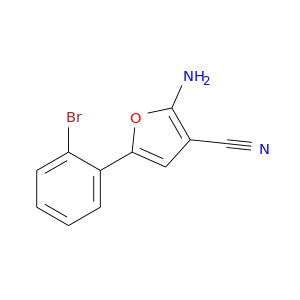
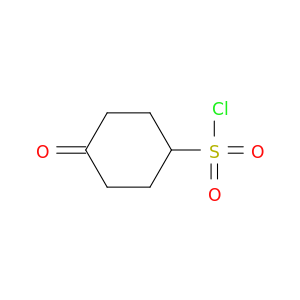
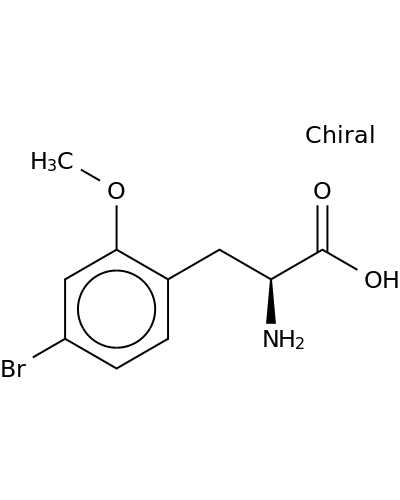
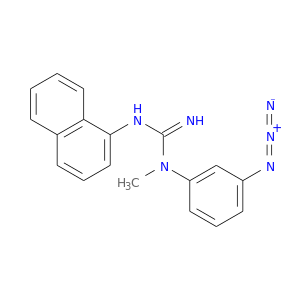
Related Products
© 2019 Angene International Limited. All rights Reserved.