200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 611-71-2
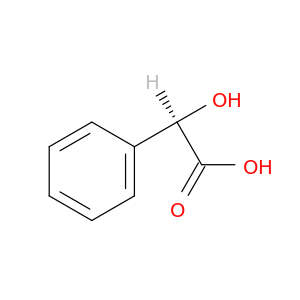
611-71-2 | D-(-)-Mandelic acid
CAS No: 611-71-2 Catalog No: AG0032DP MDL No:MFCD00064251
Product Description
Catalog Number:
AG0032DP
Chemical Name:
D-(-)-Mandelic acid
CAS Number:
611-71-2
Molecular Formula:
C8H8O3
Molecular Weight:
152.1473
MDL Number:
MFCD00064251
IUPAC Name:
(2R)-2-hydroxy-2-phenylacetic acid
InChI:
InChI=1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m1/s1
InChI Key:
IWYDHOAUDWTVEP-SSDOTTSWSA-N
SMILES:
O[C@H](c1ccccc1)C(=O)O
EC Number:
210-276-6
UNII:
PPL7YW1M9W
Properties
Complexity:
138
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
152.047g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
152.149g/mol
Monoisotopic Mass:
152.047g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
57.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.6
Literature
| Title | Journal |
|---|---|
| Investigating chiral recognizability of diastereomeric crystallization of mandelic acid and L-phenylalanine. | Journal of nanoscience and nanotechnology 20120901 |
| Feasibility of Repurposing the Polyanionic Microbicide, PPCM, for Prophylaxis against HIV Transmission during ART. | ISRN obstetrics and gynecology 20110101 |
| Metabolic engineering of the L-phenylalanine pathway in Escherichia coli for the production of S- or R-mandelic acid. | Microbial cell factories 20110101 |
| Enantiomer separation of alpha-hydroxy acids in high-performance immunoaffinity chromatography. | Journal of pharmaceutical and biomedical analysis 20080414 |
| Anti-HIV-1 activity of poly(mandelic acid) derivatives. | Biomacromolecules 20071101 |
| Intermediate analogue inhibitors of mandelate racemase: N-Hydroxyformanilide and cupferron. | Bioorganic & medicinal chemistry letters 20070101 |
| Perturbing the hydrophobic pocket of mandelate racemase to probe phenyl motion during catalysis. | Biochemistry 20050628 |
| The first preparation of enantiopure 1-methyl-7-oxabicyclo[2.2.1]heptan-2-one, a versatile chiral building block for terpenoids. | Chirality 20050201 |
| Inhibition of mandelate racemase by alpha-fluorobenzylphosphonates. | Bioorganic & medicinal chemistry letters 20030616 |
| Structural and kinetic analysis of catalysis by a thiamin diphosphate-dependent enzyme, benzoylformate decarboxylase. | Biochemistry 20030225 |
| alpha-Ketocarboxylic acid-based inhibitors of protein tyrosine phosphatases. | Bioorganic & medicinal chemistry letters 20010723 |
Related Products
© 2019 Angene International Limited. All rights Reserved.