200,000+ products from a single source!
sales@angenechem.com
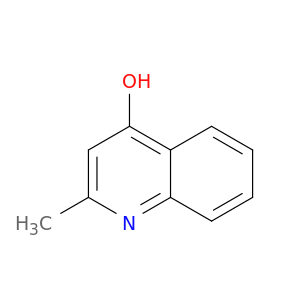
607-67-0 | 4-Hydroxy-2-methylquinoline
CAS No: 607-67-0 Catalog No: AG003LHJ MDL No:MFCD00006758
Product Description
Catalog Number:
AG003LHJ
Chemical Name:
4-Hydroxy-2-methylquinoline
CAS Number:
607-67-0
Molecular Formula:
C10H9NO
Molecular Weight:
159.1846
MDL Number:
MFCD00006758
IUPAC Name:
2-methyl-1H-quinolin-4-one
InChI:
InChI=1S/C10H9NO/c1-7-6-10(12)8-4-2-3-5-9(8)11-7/h2-6H,1H3,(H,11,12)
InChI Key:
NWINIEGDLHHNLH-UHFFFAOYSA-N
SMILES:
Cc1cc(O)c2c(n1)cccc2
EC Number:
210-140-6
NSC Number:
21483
Properties
Complexity:
232
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
159.068g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
159.188g/mol
Monoisotopic Mass:
159.068g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
29.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| Cloning and heterologous expression of the aurachin RE biosynthesis gene cluster afford a new cytochrome P450 for quinoline N-hydroxylation. | Chembiochem : a European journal of chemical biology 20130617 |
| Integrated organic-aqueous biocatalysis and product recovery for quinaldine hydroxylation catalyzed by living recombinant Pseudomonas putida. | Journal of industrial microbiology & biotechnology 20120701 |
| Regioselective aromatic hydroxylation of quinaldine by water using quinaldine 4-oxidase in recombinant Pseudomonas putida. | Journal of industrial microbiology & biotechnology 20110801 |
| AuaA, a membrane-bound farnesyltransferase from Stigmatella aurantiaca, catalyzes the prenylation of 2-methyl-4-hydroxyquinoline in the biosynthesis of aurachins. | Chembiochem : a European journal of chemical biology 20110725 |
| Copy number determination, expression analysis of genes potentially involved in replication, and stability assays of pAL1--the linear megaplasmid of Arthrobacter nitroguajacolicus Rü61a. | Microbiological research 20110120 |
| Evolutionary relationships of microbial aromatic prenyltransferases. | PloS one 20110101 |
| Endochin optimization: structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4(1H)-quinolones with antimalarial activity. | Journal of medicinal chemistry 20101014 |
| Convenient and efficient microwave-assisted synthesis of a methyl derivative of the fused indoloquinoline alkaloid cryptosanguinolentine. | Molecules (Basel, Switzerland) 20100429 |
| Iron- and 4-hydroxy-2-alkylquinoline-containing periplasmic inclusion bodies of Pseudomonas aeruginosa: a chemical analysis. | Bioorganic chemistry 20070401 |
| Spectroscopic and biochemical studies on protein variants of quinaldine 4-oxidase: Role of E736 in catalysis and effects of serine ligands on the FeSI and FeSII clusters. | Biochemistry 20061212 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Gene cluster of Arthrobacter ilicis Ru61a involved in the degradation of quinaldine to anthranilate: characterization and functional expression of the quinaldine 4-oxidase qoxLMS genes. | The Journal of biological chemistry 20030725 |
| Microbial metabolism of quinoline and related compounds. XVI. Quinaldine oxidoreductase from Arthrobacter spec. Rü 61a: a molybdenum-containing enzyme catalysing the hydroxylation at C-4 of the heterocycle. | Biological chemistry Hoppe-Seyler 19930201 |
| Microbial metabolism of quinoline and related compounds. VI. Degradation of quinaldine by Arthrobacter sp. | Biological chemistry Hoppe-Seyler 19901001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.