200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 605-02-7
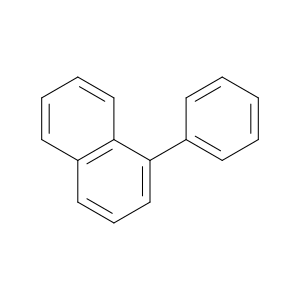
605-02-7 | 1-Phenylnaphthalene
CAS No: 605-02-7 Catalog No: AG003EML MDL No:MFCD00003983
Product Description
Catalog Number:
AG003EML
Chemical Name:
1-Phenylnaphthalene
CAS Number:
605-02-7
Molecular Formula:
C16H12
Molecular Weight:
204.2665
MDL Number:
MFCD00003983
IUPAC Name:
1-phenylnaphthalene
InChI:
InChI=1S/C16H12/c1-2-7-13(8-3-1)16-12-6-10-14-9-4-5-11-15(14)16/h1-12H
InChI Key:
IYDMICQAKLQHLA-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)c1cccc2c1cccc2
EC Number:
210-081-6
UNII:
10NXC4G45Q
NSC Number:
5257
Properties
Complexity:
214
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
204.094g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
204.272g/mol
Monoisotopic Mass:
204.094g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.8
Literature
| Title | Journal |
|---|---|
| Metal-free C-H cross-coupling toward oxygenated naphthalene-benzene linked biaryls. | Organic letters 20111202 |
| Synthesis and biological evaluation of naphthyl phenyl ethers (NPEs) as novel nonnucleoside HIV-1 reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry 20110715 |
| High hole mobility for a side-chain liquid-crystalline smectic polysiloxane exhibiting a nanosegregated structure with a terthiophene moiety. | Chemistry (Weinheim an der Bergstrasse, Germany) 20101203 |
| An efficient organocatalytic method for constructing biaryls through aromatic C-H activation. | Nature chemistry 20101201 |
| Growth of polyaromatic molecules via ion-molecule reactions: an experimental and theoretical mechanistic study. | The Journal of chemical physics 20101114 |
| 1-Phenyl-1H-naphtho-[1,2-e][1,3]oxazin-3(2H)-one. | Acta crystallographica. Section E, Structure reports online 20101001 |
| Bis(2-naphthyl-meth-yl)diphenyl-silane. | Acta crystallographica. Section E, Structure reports online 20100101 |
| Photophysical properties of 1,3,5-tris(2-naphthyl)benzene and related less-arylated compounds: experimental and theoretical investigations. | The journal of physical chemistry. A 20091231 |
| Theoretical study of the stability of the DNA duplexes modified by a series of hydrophobic base analogues. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090803 |
| Protein engineering on biphenyl dioxygenase for conferring activity to convert 7-hydroxyflavone and 5,7-dihydroxyflavone (chrysin). | Journal of bioscience and bioengineering 20080801 |
| Effects of localized triplet exciton on reactivity of photoinduced omega-bond dissociation in naphthyl phenyl ketones having pi,pi* lowest triplet (T1) states studied by laser flash photolysis. | The journal of physical chemistry. A 20060921 |
| Novel enhancement of diastereoselectivity of [2 + 2] photocycloaddition of chiral cyclohexenones to ethylene by adding naphthalenes. | The Journal of organic chemistry 20040206 |
| Photoreactions of cisoid 1,4-diphenyl-1,3-butadienes. Direct irradiation in solution and in low temperature organic glass. | Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 20040101 |
| Electronic transport in smectic liquid crystals. | Physical review. E, Statistical, nonlinear, and soft matter physics 20020401 |
| Phenylnaphthalene compounds from the subterranean part of Vitex rotundifolia and their antibacterial activity against methicillin-resistant Staphylococcus aureus. | Journal of natural products 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.