200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 603139-19-1
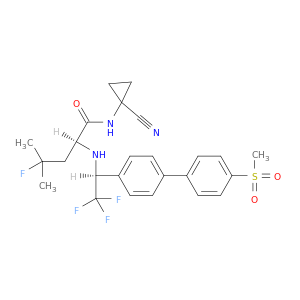
603139-19-1 | Odanacatib
CAS No: 603139-19-1 Catalog No: AG0039T3 MDL No:MFCD11042419
Product Description
Catalog Number:
AG0039T3
Chemical Name:
Odanacatib
CAS Number:
603139-19-1
Molecular Formula:
C25H27F4N3O3S
Molecular Weight:
525.5588
MDL Number:
MFCD11042419
IUPAC Name:
(2S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-[[(1S)-2,2,2-trifluoro-1-[4-(4-methylsulfonylphenyl)phenyl]ethyl]amino]pentanamide
InChI:
InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1
InChI Key:
FWIVDMJALNEADT-SFTDATJTSA-N
SMILES:
N#CC1(CC1)NC(=O)[C@H](CC(F)(C)C)N[C@H](C(F)(F)F)c1ccc(cc1)c1ccc(cc1)S(=O)(=O)C
UNII:
N673F6W2VH
Properties
Complexity:
934
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
525.171g/mol
Formal Charge:
0
Heavy Atom Count:
36
Hydrogen Bond Acceptor Count:
9
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
525.563g/mol
Monoisotopic Mass:
525.171g/mol
Rotatable Bond Count:
9
Topological Polar Surface Area:
107A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.1
Literature
| Title | Journal |
|---|---|
| Merck &Co. drops osteoporosis drug odanacatib. | Nature reviews. Drug discovery 20160929 |
| The Absolute Bioavailability and Effect of Food on the Pharmacokinetics of Odanacatib: A Stable-Label i.v./Oral Study in Healthy Postmenopausal Women. | Drug metabolism and disposition: the biological fate of chemicals 20160901 |
| Disposition and metabolism of the cathepsin K inhibitor odanacatib in humans. | Drug metabolism and disposition: the biological fate of chemicals 20140501 |
| Odanacatib, a selective cathepsin K inhibitor, demonstrates comparable pharmacodynamics and pharmacokinetics in older men and postmenopausal women. | The Journal of clinical endocrinology and metabolism 20140201 |
| Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. | The Journal of clinical endocrinology and metabolism 20130201 |
| Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20121101 |
| Pharmacokinetic benefits of 3,4-dimethoxy substitution of a phenyl ring and design of isosteres yielding orally available cathepsin K inhibitors. | Journal of medicinal chemistry 20121025 |
| Potential first-in-class osteoporosis drug speeds through trials. | Nature medicine 20120801 |
| (1R,2R)-N-(1-cyanocyclopropyl)-2-(6-methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carbonyl)cyclohexanecarboxamide (AZD4996): a potent and highly selective cathepsin K inhibitor for the treatment of osteoarthritis. | Journal of medicinal chemistry 20120726 |
| Evaluation of high-resolution peripheral quantitative computed tomography, finite element analysis and biomechanical testing in a pre-clinical model of osteoporosis: a study with odanacatib treatment in the ovariectomized adult rhesus monkey. | Bone 20120601 |
| Bone metastases: molecular mechanisms and novel therapeutic interventions. | Medicinal research reviews 20120501 |
| Use of cysteine-reactive small molecules in drug discovery for trypanosomal disease. | Expert opinion on drug discovery 20120401 |
| Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20120301 |
| Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20120301 |
| Inhibition of cathepsin K for treatment of osteoporosis. | Current osteoporosis reports 20120301 |
| Odanacatib: location and timing are everything. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20120301 |
| Quantitative determination of odanacatib in human plasma using liquid-liquid extraction followed by liquid chromatography-tandem mass spectrometry analysis. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20120215 |
| Potential role of odanacatib in the treatment of osteoporosis. | Clinical interventions in aging 20120101 |
| [Innovations in the treatment of osteoporosis]. | Deutsche medizinische Wochenschrift (1946) 20111201 |
| The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. | Bone 20111001 |
| New targets for intervention in the treatment of postmenopausal osteoporosis. | Nature reviews. Rheumatology 20110920 |
| Pharmacokinetics and metabolism in rats, dogs, and monkeys of the cathepsin k inhibitor odanacatib: demethylation of a methylsulfonyl moiety as a major metabolic pathway. | Drug metabolism and disposition: the biological fate of chemicals 20110601 |
| [Cathepsin K inhibitor: new therapy approach against osteoporosis. Pharmacological target in the osteoclast]. | MMW Fortschritte der Medizin 20110512 |
| Osteoporosis: now and the future. | Lancet (London, England) 20110409 |
| Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20110201 |
| Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20110201 |
| Difluoroethylamines as an amide isostere in inhibitors of cathepsin K. | Bioorganic & medicinal chemistry letters 20110201 |
| Discontinuation of odanacatib and other osteoporosis treatments: here today and gone tomorrow? | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20110201 |
| [Odanacatib (MK-0822)]. | Clinical calcium 20110101 |
| The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: results of a 4-week, double-blind, randomized, controlled trial. | Clinical breast cancer 20101201 |
| New approaches to treating and preventing bone metastases. | Current opinion in supportive and palliative care 20100901 |
| Emerging targets in osteoporosis disease modification. | Journal of medicinal chemistry 20100610 |
| Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20100501 |
| The resorptive apparatus of osteoclasts supports lysosomotropism and increases potency of basic versus non-basic inhibitors of cathepsin K. | Bone 20100501 |
| Exploiting new targets for old bones. | Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20100501 |
| The discovery of MK-0674, an orally bioavailable cathepsin K inhibitor. | Bioorganic & medicinal chemistry letters 20100201 |
| Peptidomimetic inhibitors of cathepsin K. | Current topics in medicinal chemistry 20100101 |
| Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. | IDrugs : the investigational drugs journal 20091201 |
| Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: a comparison of available tools. | Biological chemistry 20090901 |
| Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. | Clinical pharmacology and therapeutics 20090801 |
| [Bone remodeling: new therapeutic approaches]. | Revue medicale suisse 20090610 |
| [Prevention of joint destruction by osteoclast-targeting therapy in search of new tools, such as OPG or cathepsin K inhibitor]. | Clinical calcium 20090301 |
| A practical enantioselective synthesis of odanacatib, a potent Cathepsin K inhibitor, via triflate displacement of an alpha-trifluoromethylbenzyl triflate. | The Journal of organic chemistry 20090220 |
| Investigation of ketone warheads as alternatives to the nitrile for preparation of potent and selective cathepsin K inhibitors. | Bioorganic & medicinal chemistry letters 20090201 |
| Formulation and stability of itraconazole and odanacatib nanoparticles: governing physical parameters. | Molecular pharmaceutics 20090101 |
| Cathepsin K inhibitors as treatment of bone metastasis. | Current opinion in supportive and palliative care 20080901 |
| Molecule of the month. Odanacatib. | Drug news & perspectives 20080601 |
| The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. | Bioorganic & medicinal chemistry letters 20080201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.