200,000+ products from a single source!
sales@angenechem.com
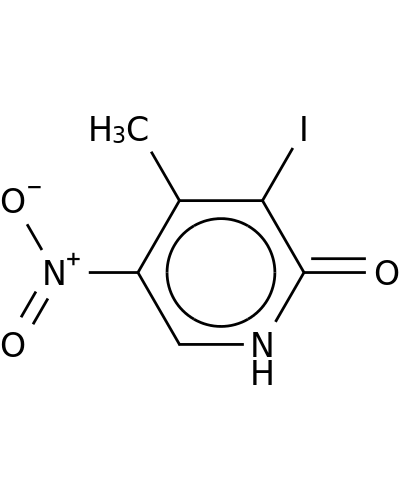
Home > Other Building Blocks > 597-52-4
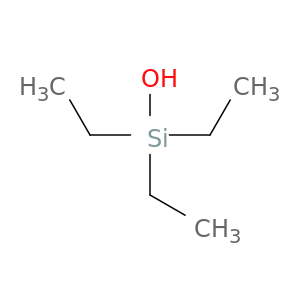
597-52-4 | Triethylsilanol
CAS No: 597-52-4 Catalog No: AG003V27 MDL No:MFCD00042640
Product Description
Catalog Number:
AG003V27
Chemical Name:
Triethylsilanol
CAS Number:
597-52-4
Molecular Formula:
C6H16OSi
Molecular Weight:
132.2761
MDL Number:
MFCD00042640
IUPAC Name:
triethyl(hydroxy)silane
InChI:
InChI=1S/C6H16OSi/c1-4-8(7,5-2)6-3/h7H,4-6H2,1-3H3
InChI Key:
WVMSIBFANXCZKT-UHFFFAOYSA-N
SMILES:
CC[Si](CC)(CC)O
EC Number:
209-903-6
Properties
Complexity:
51.3
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
132.097g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
132.278g/mol
Monoisotopic Mass:
132.097g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
20.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Cyclodextrins selectively modified on both rims using an O-3-debenzylative post-functionalisation, a consequence of the Sorrento meeting. | Carbohydrate research 20120715 |
| Total syntheses of ent-heliespirones A and C. | The Journal of organic chemistry 20120106 |
| Ring cleavage reactions of methyl α-D-allopyranoside derivatives with phenylboron dichloride and triethylsilane. | Molecules (Basel, Switzerland) 20111213 |
| Direct, one-pot reductive alkylation of anilines with functionalized acetals mediated by triethylsilane and TFA. Straightforward route for unsymmetrically substituted ethylenediamine. | The Journal of organic chemistry 20110121 |
| Preparation of iron and gold silicide nanodomains on silicon (111) by the reaction of gold, iron-gold core-shell, and alloy nanoparticles with triethylsilane. | ACS applied materials & interfaces 20100801 |
| Iridium-catalyzed (Z)-trialkylsilylation of terminal olefins. | The Journal of organic chemistry 20100305 |
| Application of triethylsilane and palladium-charcoal-induced reductions in the synthesis of Fmoc-glutamic acid analogues. | Advances in experimental medicine and biology 20090101 |
| Triethylsilane as a mild and efficient reducing agent for the preparation of alkanethiol-capped gold nanoparticles. | Chemical communications (Cambridge, England) 20080907 |
| Solute-solvent complex kinetics and thermodynamics probed by 2D-IR vibrational echo chemical exchange spectroscopy. | The journal of physical chemistry. B 20080821 |
| One-pot synthesis of oligosaccharides by combining reductive openings of benzylidene acetals and glycosylations. | Organic letters 20080807 |
| (2+2) cycloaddition reaction of alkyl enol ethers with acrylates by in situ generated silyl triflic imide catalyst. | Chemical & pharmaceutical bulletin 20080801 |
| Vinylation of aromatic halides using inexpensive organosilicon reagents. Illustration of design of experiment protocols. | Journal of the American Chemical Society 20080319 |
| Triethylsilanol: molecular conformations and role of the hydrogen-bonding oligomerization in its vibrational spectra. | The journal of physical chemistry. A 20080221 |
| Pd-C-induced catalytic transfer hydrogenation with triethylsilane. | The Journal of organic chemistry 20070817 |
| Nucleophilic aromatic substitution using Et3SiH/cat. t-Bu-P4 as a system for nucleophile activation. | Chemical communications (Cambridge, England) 20070614 |
| Construction of fused bis(pyran) units from enones via a hydrosilylation-dihydroxylation-acetalization-reduction sequence. | Chemical communications (Cambridge, England) 20061228 |
| Pd-catalyzed silicon hydride reductions of aromatic and aliphatic nitro groups. | Organic letters 20051027 |
| Stereoselective synthesis of 4'-carbon-substituted xylofuranosyladenines. | Nucleic acids symposium series (2004) 20040101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.