200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 58436-28-5
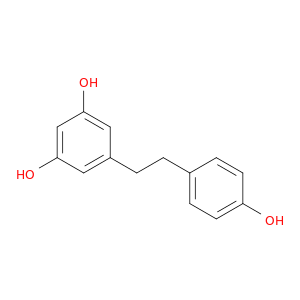
58436-28-5 | Dihydroresveratrol
CAS No: 58436-28-5 Catalog No: AG0038MU MDL No:MFCD25973128
Product Description
Catalog Number:
AG0038MU
Chemical Name:
Dihydroresveratrol
CAS Number:
58436-28-5
Molecular Formula:
C14H14O3
Molecular Weight:
230.2592
MDL Number:
MFCD25973128
IUPAC Name:
5-[2-(4-hydroxyphenyl)ethyl]benzene-1,3-diol
InChI:
InChI=1S/C14H14O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h3-9,15-17H,1-2H2
InChI Key:
HITJFUSPLYBJPE-UHFFFAOYSA-N
SMILES:
Oc1ccc(cc1)CCc1cc(O)cc(c1)O
EC Number:
611-651-0
NSC Number:
723534
Properties
Complexity:
214
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
230.094g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
230.263g/mol
Monoisotopic Mass:
230.094g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
60.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.1
Literature
| Title | Journal |
|---|---|
| Hydroxystilbenes and methoxystilbenes activate human aryl hydrocarbon receptor and induce CYP1A genes in human hepatoma cells and human hepatocytes. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20170501 |
| Effects of long-term consumption of low doses of resveratrol on diet-induced mild hypercholesterolemia in pigs: a transcriptomic approach to disease prevention. | The Journal of nutritional biochemistry 20120701 |
| The journey of resveratrol from yeast to human. | Aging 20120301 |
| Optimizing thiadiazole analogues of resveratrol versus three chemopreventive targets. | Bioorganic & medicinal chemistry 20120101 |
| Resveratrol, but not dihydroresveratrol, induces premature senescence in primary human fibroblasts. | Age (Dordrecht, Netherlands) 20111201 |
| Metabolites and tissue distribution of resveratrol in the pig. | Molecular nutrition & food research 20110801 |
| A study of resveratrol-copper complexes by electrospray ionization mass spectrometry and density functional theory calculations. | Rapid communications in mass spectrometry : RCM 20110227 |
| Investigation of piceid metabolites in rat by liquid chromatography tandem mass spectrometry. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20110101 |
| Bioavailability of resveratrol. | Annals of the New York Academy of Sciences 20110101 |
| Dihydro-resveratrol--a potent dietary polyphenol. | Bioorganic & medicinal chemistry letters 20101015 |
| trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. | Journal of agricultural and food chemistry 20100714 |
| Determination of dihydroresveratrol in rat plasma by HPLC. | Journal of agricultural and food chemistry 20100623 |
| A theoretical study of the structure-radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. | European journal of medicinal chemistry 20100301 |
| Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. | Journal of medicinal chemistry 20090813 |
| Interaction of dietary resveratrol with animal-associated bacteria. | FEMS microbiology letters 20090801 |
| Biocatalytic production of acyclic bis[bibenzyls] from dihydroresveratrol by crude Momordica charantia peroxidase. | Chemistry & biodiversity 20090801 |
| [Studies on constituents of Dendrobium gratiosissimum]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20070401 |
| Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20051227 |
| Phytotoxic activity of bibenzyl derivatives from the orchid Epidendrum rigidum. | Journal of agricultural and food chemistry 20050810 |
| Resveratrol derivatives and their role as potassium channels modulators. | Journal of natural products 20040301 |
| Spasmolytic effects, mode of action, and structure-activity relationships of stilbenoids from Nidema boothii. | Journal of natural products 20040201 |
| Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. | The Journal of biological chemistry 20010622 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.