200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 5469-16-9
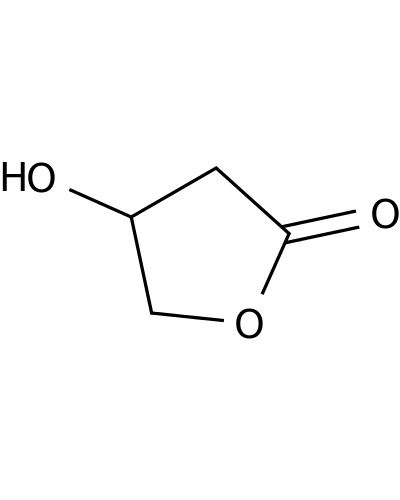
5469-16-9 | 4-Hydroxydihydrofuran-2(3H)-one
CAS No: 5469-16-9 Catalog No: AG003BDP MDL No:MFCD00090014
Product Description
Catalog Number:
AG003BDP
Chemical Name:
4-Hydroxydihydrofuran-2(3H)-one
CAS Number:
5469-16-9
Molecular Formula:
C4H6O3
Molecular Weight:
102.0886
MDL Number:
MFCD00090014
IUPAC Name:
4-hydroxyoxolan-2-one
InChI:
InChI=1S/C4H6O3/c5-3-1-4(6)7-2-3/h3,5H,1-2H2
InChI Key:
FUDDLSHBRSNCBV-UHFFFAOYSA-N
SMILES:
OC1CC(=O)OC1
NSC Number:
26907
Properties
Complexity:
88.9
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
102.032g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
102.089g/mol
Monoisotopic Mass:
102.032g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
46.5A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.8
Literature
| Title | Journal |
|---|---|
| Honaucins A-C, potent inhibitors of inflammation and bacterial quorum sensing: synthetic derivatives and structure-activity relationships. | Chemistry & biology 20120525 |
| Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. | Pharmaceutical biology 20110701 |
| Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile--identification of novel compounds. | Talanta 20110115 |
| Uses and production of chiral 3-hydroxy-gamma-butyrolactones and structurally related chemicals. | Applied microbiology and biotechnology 20091001 |
| A chemoenzymatic approach to the synthesis of enantiomerically pure (S)-3-hydroxy-gamma-butyrolactone. | Applied microbiology and biotechnology 20080601 |
| Isolation and properties of a levo-lactonase from Fusarium proliferatum ECU2002: a robust biocatalyst for production of chiral lactones. | Applied microbiology and biotechnology 20070701 |
| Improvement on production of (R)-4-chloro-3-hydroxybutyrate and (S)-3-hydroxy-gamma-butyrolactone with recombinant Escherichia coli cells. | Journal of bioscience and bioengineering 20060201 |
| New synthesis of chiral 1,3-oxazinan-2-ones from carbohydrate derivatives. | The Journal of organic chemistry 20050121 |
| Physiological significance of 2-buten-4-olide (2-B4O), an endogenous satiety substance increased in the fasted state. | Experimental biology and medicine (Maywood, N.J.) 20031101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.