200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 546-06-5
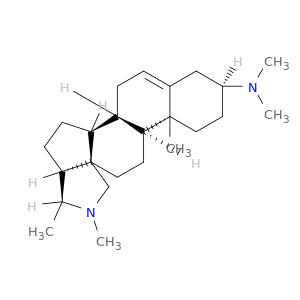
546-06-5 | Conessine
CAS No: 546-06-5 Catalog No: AG003P1J MDL No:
Product Description
Catalog Number:
AG003P1J
Chemical Name:
Conessine
CAS Number:
546-06-5
Molecular Formula:
C24H40N2
Molecular Weight:
356.5878
IUPAC Name:
(1R,2S,5S,6S,9R,12S,13R,16S)-N,N,6,7,13-pentamethyl-7-azapentacyclo[10.8.0.02,9.05,9.013,18]icos-18-en-16-amine
InChI:
InChI=1S/C24H40N2/c1-16-20-8-9-22-19-7-6-17-14-18(25(3)4)10-12-23(17,2)21(19)11-13-24(20,22)15-26(16)5/h6,16,18-22H,7-15H2,1-5H3/t16-,18-,19+,20+,21-,22-,23-,24-/m0/s1
InChI Key:
GPLGAQQQNWMVMM-MYAJQUOBSA-N
SMILES:
CN([C@H]1CC[C@]2(C(=CC[C@@H]3[C@@H]2CC[C@]24[C@H]3CC[C@@H]4[C@@H](N(C2)C)C)C1)C)C
EC Number:
208-897-2
UNII:
EZ38J9BBDF
Properties
Complexity:
609
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
8
Defined Bond Stereocenter Count:
0
Exact Mass:
356.319g/mol
Formal Charge:
0
Heavy Atom Count:
26
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
356.598g/mol
Monoisotopic Mass:
356.319g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
6.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.9
Literature
| Title | Journal |
|---|---|
| International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. | Pharmacological reviews 20150701 |
| Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. | Malaria journal 20130101 |
| Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. | Bioorganic & medicinal chemistry 20121115 |
| Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure-activity relationships. | Life sciences 20120614 |
| Antimicrobial activity of the methanolic bark extract of Holarrhena pubescens (Buch. Ham), its fractions and the pure compound conessine. | Natural product research 20120101 |
| Novel chalcone-based fluorescent human histamine h(3) receptor ligands as pharmacological tools. | Frontiers in systems neuroscience 20120101 |
| Cloning, expression, characterization and inhibition studies on trypanothione synthetase, a drug target enzyme, from Leishmania donovani. | Biological chemistry 20111201 |
| Anti-malarial activity of Holarrhena antidysenterica and Viola canescens, plants traditionally used against malaria in the Garhwal region of north-west Himalaya. | Malaria journal 20110101 |
| Identification of novel functional inhibitors of acid sphingomyelinase. | PloS one 20110101 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Acute cardiac toxicity of nerium oleander/indicum poisoning (kaner) poisoning. | Heart views : the official journal of the Gulf Heart Association 20100101 |
| Design of a new histamine H3 receptor antagonist chemotype: (3aR,6aR)-5-alkyl-1-aryl-octahydropyrrolo[3,4-b]pyrroles, synthesis, and structure-activity relationships. | Journal of medicinal chemistry 20090813 |
| Piperidine variations in search for non-imidazole histamine H(3) receptor ligands. | Bioorganic & medicinal chemistry 20080915 |
| The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. | Journal of medicinal chemistry 20080911 |
| Novel H3 receptor antagonists with improved pharmacokinetic profiles. | Bioorganic & medicinal chemistry letters 20080715 |
| The use of natural product scaffolds as leads in the search for trypanothione reductase inhibitors. | Bioorganic & medicinal chemistry 20080715 |
| A new family of H3 receptor antagonists based on the natural product Conessine. | Bioorganic & medicinal chemistry letters 20080215 |
| High-performance thin layer chromatography method for estimation of conessine in herbal extract and pharmaceutical dosage formulations. | Journal of pharmaceutical and biomedical analysis 20080122 |
| Chromatographic analysis of Kutajarista--an ayurvedic polyherbal formulation. | Phytochemical analysis : PCA 20080101 |
| Development and validation of a visible absorption densitometry method for quantitation of conessine in Holarrhena antidysenterica (Kurchi). | Journal of AOAC International 20080101 |
| Antagonist affinity measurements at the Gi-coupled human histamine H3 receptor expressed in CHO cells. | BMC pharmacology 20080101 |
| Steroidal alkaloids from Holarrhena antidysenterica (L.) WALL. | Chemical & pharmaceutical bulletin 20070601 |
| A new family of histamine H3 receptor antagonists based on a natural product: discovery, SAR, and properties of the series. | Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20070401 |
| Antibacterial activities of the extracts and conessine from Holarrhena floribunda G. Don. (Apocynaceae). | African journal of traditional, complementary, and alternative medicines : AJTCAM 20070101 |
| Prediction of estrogen receptor agonists and characterization of associated molecular descriptors by statistical learning methods. | Journal of molecular graphics & modelling 20061101 |
| Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. | Bioorganic & medicinal chemistry letters 20050516 |
| Highly enantioselective construction of fused pyrrolidine systems that contain a quaternary stereocenter: concise formal synthesis of (+)-conessine. | Angewandte Chemie (International ed. in English) 20040503 |
| Steroid hormone activity of flavonoids and related compounds. | Breast cancer research and treatment 20000701 |
| Déjà vu guides the way to new antimicrobial steroid. | Science (New York, N.Y.) 19930219 |
| Squalamine: an aminosterol antibiotic from the shark. | Proceedings of the National Academy of Sciences of the United States of America 19930215 |
Related Products
© 2019 Angene International Limited. All rights Reserved.