200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 520-03-6
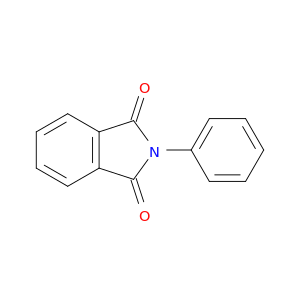
520-03-6 | 2-Phenylisoindole-1,3-dione
CAS No: 520-03-6 Catalog No: AG003HVM MDL No:MFCD00023044
Product Description
Catalog Number:
AG003HVM
Chemical Name:
2-Phenylisoindole-1,3-dione
CAS Number:
520-03-6
Molecular Formula:
C14H9NO2
Molecular Weight:
223.2268
MDL Number:
MFCD00023044
IUPAC Name:
2-phenylisoindole-1,3-dione
InChI:
InChI=1S/C14H9NO2/c16-13-11-8-4-5-9-12(11)14(17)15(13)10-6-2-1-3-7-10/h1-9H
InChI Key:
MFUPLJQNEXUUDW-UHFFFAOYSA-N
SMILES:
O=C1N(c2ccccc2)C(=O)c2c1cccc2
EC Number:
208-282-9
UNII:
LA29QA196M
NSC Number:
2769
Properties
Complexity:
309
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
223.063g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
223.231g/mol
Monoisotopic Mass:
223.063g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
37.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| Inhibition of monoamine oxidase by C5-substituted phthalimide analogues. | Bioorganic & medicinal chemistry 20110815 |
| Identification of the binding modes of N-phenylphthalimides inhibiting bacterial thymidylate synthase through X-ray crystallography screening. | Journal of medicinal chemistry 20110811 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Development of tryptase inhibitors derived from thalidomide. | Bioorganic & medicinal chemistry 20100715 |
| Synthesis and in vitro evaluation of N-substituted maleimide derivatives as selective monoglyceride lipase inhibitors. | Journal of medicinal chemistry 20091210 |
| Glycogen phosphorylase a inhibitors with a phenethylphenylphthalimide skeleton derived from thalidomide-related alpha-glucosidase inhibitors and liver X receptor antagonists. | Biological & pharmaceutical bulletin 20090901 |
| A new polymorph of N-phenyl-phthalimide. | Acta crystallographica. Section E, Structure reports online 20090301 |
| Synthesis of N-phenylphthalimide derivatives as alpha-glucosidase inhibitors. | Archives of pharmacal research 20071201 |
| Selective inhibition of carboxylesterases by isatins, indole-2,3-diones. | Journal of medicinal chemistry 20070419 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Angiogenesis inhibitors derived from thalidomide. | Bioorganic & medicinal chemistry letters 20051215 |
| Design, synthesis and antiinflammatory activity of novel phthalimide derivatives, structurally related to thalidomide. | Bioorganic & medicinal chemistry letters 20050215 |
| Tubulin-polymerization inhibitors derived from thalidomide. | Bioorganic & medicinal chemistry letters 20050117 |
| N-phenylphthalimide-type cyclooxygenase (COX) inhibitors derived from thalidomide: substituent effects on subtype selectivity. | Chemical & pharmaceutical bulletin 20040801 |
| Synthesis and anti-inflammatory activity of phthalimide derivatives, designed as new thalidomide analogues. | Bioorganic & medicinal chemistry 20020901 |
| Thalidomide and its analogues as cyclooxygenase inhibitors. | Bioorganic & medicinal chemistry letters 20020408 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.