200,000+ products from a single source!
sales@angenechem.com
Home > Sulfanilamide > 50487-72-4
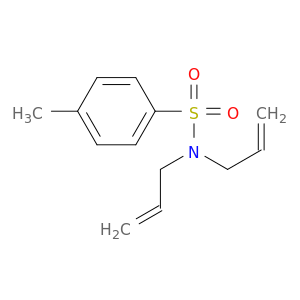
50487-72-4 | N,N-DIALLYL-4-METHYLBENZENESULFONAMIDE
CAS No: 50487-72-4 Catalog No: AG00DG1H MDL No:MFCD00226469
Product Description
Catalog Number:
AG00DG1H
Chemical Name:
N,N-DIALLYL-4-METHYLBENZENESULFONAMIDE
CAS Number:
50487-72-4
Molecular Formula:
C13H17NO2S
Molecular Weight:
251.3446
MDL Number:
MFCD00226469
IUPAC Name:
4-methyl-N,N-bis(prop-2-enyl)benzenesulfonamide
InChI:
InChI=1S/C13H17NO2S/c1-4-10-14(11-5-2)17(15,16)13-8-6-12(3)7-9-13/h4-9H,1-2,10-11H2,3H3
InChI Key:
OKXHMBXWZHCSMR-UHFFFAOYSA-N
SMILES:
C=CCN(S(=O)(=O)c1ccc(cc1)C)CC=C
Properties
Complexity:
338
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
251.098g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
251.344g/mol
Monoisotopic Mass:
251.098g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
45.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.7
Literature
| Title | Journal |
|---|---|
| Ring-closing metathesis in aqueous micellar medium. | Chemistry (Weinheim an der Bergstrasse, Germany) 20120116 |
| Artificial metalloenzymes for olefin metathesis based on the biotin-(strept)avidin technology. | Chemical communications (Cambridge, England) 20111128 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Novel ruthenium-based metathesis catalysts containing electron-withdrawing ligands: synthesis, immobilization, and reactivity. | The Journal of organic chemistry 20050610 |
Related Products
© 2019 Angene International Limited. All rights Reserved.