200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 4972-31-0
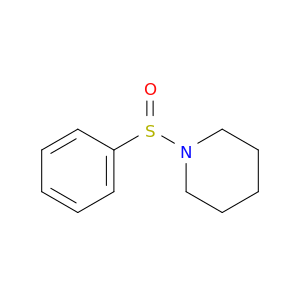
4972-31-0 | 1-(Phenylsulfinyl)piperidine
CAS No: 4972-31-0 Catalog No: AG003D3W MDL No:MFCD05155800
Product Description
Catalog Number:
AG003D3W
Chemical Name:
1-(Phenylsulfinyl)piperidine
CAS Number:
4972-31-0
Molecular Formula:
C11H15NOS
Molecular Weight:
209.3079
MDL Number:
MFCD05155800
IUPAC Name:
1-(benzenesulfinyl)piperidine
InChI:
InChI=1S/C11H15NOS/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1,3-4,7-8H,2,5-6,9-10H2
InChI Key:
LBRJCAJLGAXDKP-UHFFFAOYSA-N
SMILES:
O=S(c1ccccc1)N1CCCCC1
Properties
Complexity:
196
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
209.087g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
209.307g/mol
Monoisotopic Mass:
209.087g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
39.5A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| Highly stereoselective synthesis of alpha-D-mannopyranosyl phosphosugars. | The Journal of organic chemistry 20091218 |
| Pre-activation of fully acetylated dodecyl thioglycosides with BSP-Tf2O led to efficient glycosylation at low temperature. | Carbohydrate research 20090217 |
| Is donor-acceptor hydrogen bonding necessary for 4,6-O-benzylidene-directed beta-mannopyranosylation? Stereoselective synthesis of beta-C-mannopyranosides and alpha-C-glucopyranosides. | Organic letters 20081106 |
| 4,6-O-benzylidene-directed beta-mannopyranosylation and alpha-glucopyranosylation: the 2-deoxy-2-fluoro and 3-deoxy-3-fluoro series of donors and the importance of the O2-C2-C3-O3 interaction. | The Journal of organic chemistry 20070302 |
| On the use of 3,5-O-benzylidene and 3,5-O-(di-tert-butylsilylene)-2-O-benzylarabinothiofuranosides and their sulfoxides as glycosyl donors for the synthesis of beta-arabinofuranosides: importance of the activation method. | The Journal of organic chemistry 20070302 |
| Synthesis of n-octyl 2,6-dideoxy-alpha-L-lyxo-hexopyranosyl-(1-->2)-3-amino-3-deoxy-beta-D-galactopyranoside, an analog of the H-disaccharide antigen. | Carbohydrate research 20060724 |
| Stereocontrolled formation of beta-glucosides and related linkages in the absence of neighboring group participation: influence of a trans-fused 2,3-O-carbonate group. | The Journal of organic chemistry 20050902 |
| Direct synthesis of the beta-l-rhamnopyranosides. | Organic letters 20030306 |
| Solid-phase synthesis of beta-mannosides. | Journal of the American Chemical Society 20020731 |
| Synthesis of the Salmonella type E(1) core trisaccharide as a probe for the generality of 1-(benzenesulfinyl)piperidine/triflic anhydride combination for glycosidic bond formation from thioglycosides. | The Journal of organic chemistry 20020712 |
| 1-Benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride: a potent combination of shelf-stable reagents for the low-temperature conversion of thioglycosides to glycosyl triflates and for the formation of diverse glycosidic linkages. | Journal of the American Chemical Society 20010919 |
Related Products
© 2019 Angene International Limited. All rights Reserved.