200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 490-31-3
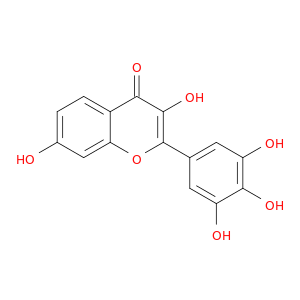
490-31-3 | 4H-1-Benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)
CAS No: 490-31-3 Catalog No: AG00391A MDL No:MFCD00016783
Product Description
Catalog Number:
AG00391A
Chemical Name:
4H-1-Benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)
CAS Number:
490-31-3
Molecular Formula:
C15H10O7
Molecular Weight:
302.2357
MDL Number:
MFCD00016783
IUPAC Name:
3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
InChI:
InChI=1S/C15H10O7/c16-7-1-2-8-11(5-7)22-15(14(21)12(8)19)6-3-9(17)13(20)10(18)4-6/h1-5,16-18,20-21H
InChI Key:
SOEDEYVDCDYMMH-UHFFFAOYSA-N
SMILES:
Oc1ccc2c(c1)oc(c(c2=O)O)c1cc(O)c(c(c1)O)O
EC Number:
207-709-6
UNII:
KJ6DBC4U7E
Properties
Complexity:
476
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
302.043g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
7
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
302.238g/mol
Monoisotopic Mass:
302.043g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
127A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.6
Literature
| Title | Journal |
|---|---|
| Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. | Toxicological sciences : an official journal of the Society of Toxicology 20180701 |
| Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. | Bioorganic & medicinal chemistry 20121115 |
| Synthesis and biological evaluation of novel flavonols as potential anti-prostate cancer agents. | European journal of medicinal chemistry 20120801 |
| Polyphenolic profile as a useful tool to identify the wood used in wine aging. | Analytica chimica acta 20120630 |
| Flavonoids as inhibitors of human CD38. | Bioorganic & medicinal chemistry letters 20110701 |
| The biochemical and cellular basis for nutraceutical strategies to attenuate neurodegeneration in Parkinson's disease. | International journal of molecular sciences 20110101 |
| Identification by high-throughput screening of inhibitors of Schistosoma mansoni NAD(+) catabolizing enzyme. | Bioorganic & medicinal chemistry 20101115 |
| Relationships between structures of hydroxyflavones and their antioxidative effects. | Bioorganic & medicinal chemistry letters 20100915 |
| Binding of the bioflavonoid robinetin with model membranes and hemoglobin: Inhibition of lipid peroxidation and protein glycosylation. | Journal of photochemistry and photobiology. B, Biology 20100121 |
| Modulation of multidrug resistance protein 1 (MRP1/ABCC1)-mediated multidrug resistance by bivalent apigenin homodimers and their derivatives. | Journal of medicinal chemistry 20090910 |
| Effect of beta-cyclodextrin nanocavity confinement on the photophysics of robinetin. | Journal of photochemistry and photobiology. B, Biology 20071214 |
| An in vitro and in silico study on the flavonoid-mediated modulation of the transport of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) through Caco-2 monolayers. | Toxicology and applied pharmacology 20061201 |
| Antimicrobial activity of flavonoids. | International journal of antimicrobial agents 20051101 |
| Allelopathic potential of Robinia pseudo-acacia L. | Journal of chemical ecology 20050901 |
| Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. | Biochemical pharmacology 20050215 |
| Effects of selected flavonoids and carotenoids on drug accumulation and apoptosis induction in multidrug-resistant colon cancer cells expressing MDR1/LRP. | In vivo (Athens, Greece) 20050101 |
| Kinetic study of flavonoid reactions with stable radicals. | Journal of agricultural and food chemistry 20040519 |
| Study of freeze-dried quercetin-cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. | Journal of pharmaceutical and biomedical analysis 20040204 |
| Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. | Mutation research 20030909 |
| Application of the electrotopological state index to QSAR analysis of flavone derivatives as HIV-1 integrase inhibitors. | Pharmaceutical research 19961201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.