200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 487-60-5
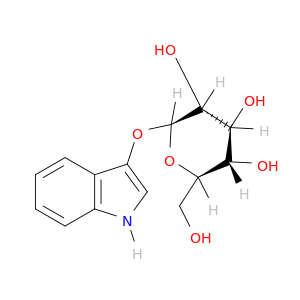
487-60-5 | 3-Indolyl-b-D-glucopyranoside
CAS No: 487-60-5 Catalog No: AG003JL4 MDL No:MFCD00047169
Product Description
Catalog Number:
AG003JL4
Chemical Name:
3-Indolyl-b-D-glucopyranoside
CAS Number:
487-60-5
Molecular Formula:
C14H17NO6
Molecular Weight:
295.2879
MDL Number:
MFCD00047169
IUPAC Name:
(2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-(1H-indol-3-yloxy)oxane-3,4,5-triol
InChI:
InChI=1S/C14H17NO6/c16-6-10-11(17)12(18)13(19)14(21-10)20-9-5-15-8-4-2-1-3-7(8)9/h1-5,10-19H,6H2/t10-,11-,12+,13-,14-/m1/s1
InChI Key:
XVARCVCWNFACQC-RKQHYHRCSA-N
SMILES:
OC[C@H]1O[C@@H](Oc2c[nH]c3c2cccc3)[C@@H]([C@H]([C@@H]1O)O)O
EC Number:
610-433-2
UNII:
N187WK1Y1J
Properties
Complexity:
356
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
295.106g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
295.291g/mol
Monoisotopic Mass:
295.106g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
115A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.1
Literature
| Title | Journal |
|---|---|
| Alkaloids from the root of Isatis indigotica. | Journal of natural products 20120622 |
| Formation of the indigo precursor indican in genetically engineered tobacco plants and cell cultures. | Plant biotechnology journal 20070101 |
| Beta-glucosidase-catalyzed hydrolysis of indican from leaves of Polygonum tinctorium. | Biotechnology progress 20020101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.