200,000+ products from a single source!
sales@angenechem.com
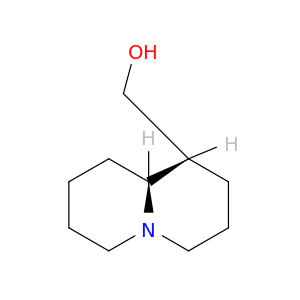
486-70-4 | ((1R,9aR)-Octahydro-1H-quinolizin-1-yl)methanol
CAS No: 486-70-4 Catalog No: AG003BAW MDL No:MFCD01083034
Product Description
Catalog Number:
AG003BAW
Chemical Name:
((1R,9aR)-Octahydro-1H-quinolizin-1-yl)methanol
CAS Number:
486-70-4
Molecular Formula:
C10H19NO
Molecular Weight:
169.2640
MDL Number:
MFCD01083034
IUPAC Name:
[(1R,9aR)-2,3,4,6,7,8,9,9a-octahydro-1H-quinolizin-1-yl]methanol
InChI:
InChI=1S/C10H19NO/c12-8-9-4-3-7-11-6-2-1-5-10(9)11/h9-10,12H,1-8H2/t9-,10+/m0/s1
InChI Key:
HDVAWXXJVMJBAR-VHSXEESVSA-N
SMILES:
OC[C@@H]1CCCN2[C@@H]1CCCC2
EC Number:
207-638-0
UNII:
33BAJ73U1F
NSC Number:
21723
Properties
Complexity:
149
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
169.147g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
169.268g/mol
Monoisotopic Mass:
169.147g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
23.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. | Bioorganic & medicinal chemistry 20121115 |
| Synthesis and comparison of antiplasmodial activity of (+), (-) and racemic 7-chloro-4-(N-lupinyl)aminoquinoline. | Bioorganic & medicinal chemistry 20121001 |
| [Derivatives of lupinin and epilupinin as ligands of various cholinesterases]. | Zhurnal evoliutsionnoi biokhimii i fiziologii 20120101 |
| [Isomeric derivatives of lupinine and epilupinine--organophosphorus inhibitors of cholinesterases]. | Ukrains'kyi biokhimichnyi zhurnal (1999 ) 20120101 |
| [Reversible lupininin inhibitors of cholinesterases of mammalian blood and of optical ganglia of individuals of the commander squid Berryteuthis magister from different zones of species areal]. | Zhurnal evoliutsionnoi biokhimii i fiziologii 20120101 |
| A novel sesquiterpene acid and an alkaloid from leaves of the Eastern Nigeria mistletoe, Loranthus micranthus with potent immunostimulatory activity on C57BL6 mice splenocytes and CD69 molecule. | Pharmaceutical biology 20111201 |
| Total syntheses of (-) epilupinine and (-)-tashiromine using imino-aldol reactions. | Organic letters 20110805 |
| Total synthesis of (+)-epilupinine via an intramolecular nitrile oxide-alkene cycloaddition. | The Journal of organic chemistry 20110107 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Short access to (+)-lupinine and (+)-epiquinamide via double hydroformylation. | Organic letters 20100205 |
| Epiquinamide: a poison that wasn't from a frog that was. | Journal of natural products 20090227 |
| Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. | Journal of ethnobiology and ethnomedicine 20090101 |
| A direct stereoselective approach to trans-2,3-disubstituted piperidines: application in the synthesis of 2-Epi-CP-99,994 and (+)-epilupinine. | Organic letters 20080619 |
| Analogues of amphibian alkaloids: total synthesis of (5R,8S,8aS)-(-)-8-methyl-5-pentyloctahydroindolizine (8-epi-indolizidine 209B) and [(1S,4R,9aS)-(-)-4-pentyloctahydro-2H-quinolizin-1-yl]methanol. | Beilstein journal of organic chemistry 20080101 |
| Cholinesterase hydrolysis of substituted lupinine benzoates. | Doklady. Biochemistry and biophysics 20080101 |
| Bactericidal and fungicidal activities of Calia secundiflora (Ort.) Yakovlev. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20080101 |
| Phytochemical differences between Calia secundiflora (Leguminosae) growing at two sites in Mexico. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20060101 |
| Cascade iminium ion reactions for the facile synthesis of quinolizidines. Concise syntheses of (+/-)-epilupinine and (-)-epimyrtine. | Organic letters 20050512 |
| [Synthesis of lupinine derivatives of flavonoids]. | Bioorganicheskaia khimiia 20050101 |
| Double ring-closing metathesis reaction of nitrogen-containing tetraenes: efficient construction of bicyclic alkaloid skeletons and synthetic application to four stereoisomers of lupinine and their derivatives. | Chemistry (Weinheim an der Bergstrasse, Germany) 20040705 |
| [Application of bisalkaloid derivatives of dicarboxylic acids based on lupinine, anabasine and cytisine as cholinesterase inhibitors of various origin]. | Zhurnal evoliutsionnoi biokhimii i fiziologii 20040101 |
| Bisalkaloid derivatives of dicarboxylic acids on the basis of lupinine, anabasine, and cytisine as reversible cholinesterase inhibitors. | Doklady. Biochemistry and biophysics 20030101 |
| Thesinine-4'-O-beta-D-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. | Phytochemistry 20020601 |
| Indolizidine and quinolizidine alkaloids. | Natural product reports 20011001 |
| 2-(4-R-phenoxy/phenylthio)alkanoic esters of l-lupinine. | Farmaco (Societa chimica italiana : 1989) 20010301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.