200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 4746-97-8
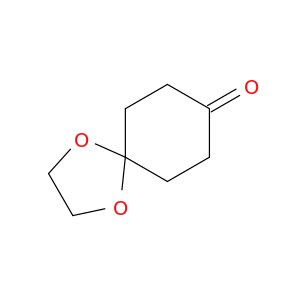
4746-97-8 | 1,4-Cyclohexanedione Monoethylene Acetal
CAS No: 4746-97-8 Catalog No: AG0032MH MDL No:MFCD00010214
Product Description
Catalog Number:
AG0032MH
Chemical Name:
1,4-Cyclohexanedione Monoethylene Acetal
CAS Number:
4746-97-8
Molecular Formula:
C8H12O3
Molecular Weight:
156.1791
MDL Number:
MFCD00010214
IUPAC Name:
1,4-dioxaspiro[4.5]decan-8-one
InChI:
InChI=1S/C8H12O3/c9-7-1-3-8(4-2-7)10-5-6-11-8/h1-6H2
InChI Key:
VKRKCBWIVLSRBJ-UHFFFAOYSA-N
SMILES:
O=C1CCC2(CC1)OCCO2
EC Number:
610-352-2
Properties
Complexity:
158
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
156.079g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
156.181g/mol
Monoisotopic Mass:
156.079g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
35.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.1
Literature
| Title | Journal |
|---|---|
| 13,13-Dimethyl-des-C,D analogues of (20S)-1α,25-dihydroxy-2-methylene-19-norvitamin D₃ (2MD): total synthesis, docking to the VDR, and biological evaluation. | Bioorganic & medicinal chemistry 20111201 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| 1-desoxy analog of 2MD: synthesis and biological activity of (20S)-25-hydroxy-2-methylene-19-norvitamin D3. | The Journal of steroid biochemistry and molecular biology 20100701 |
| Enantio- and diastereoselective total synthesis of (+)-panepophenanthrin, a ubiquitin-activating enzyme inhibitor, and biological properties of its new derivatives. | Chemistry, an Asian journal 20061218 |
| Studies on a total synthesis of the microbial immunosuppresive agent FR901483. | The Journal of organic chemistry 20060303 |
| pi-Facial stereoselectivity in Diels-Alder cycloadditions to 1-oxaspiro[4.5]deca-6,9-dien-8-one. The strong directive effect of ether oxygen in a cross-conjugated ketone setting. | Organic letters 20030724 |
| Aromatization of 1,6,7,7a-tetrahydro-2H-indol-2-ones by a novel process. Preparation of key-intermediate methyl 1-benzyl-5-methoxy-1H-indole-3-acetate and the syntheses of serotonin, melatonin, and bufotenin. | The Journal of organic chemistry 20020405 |
Related Products
© 2019 Angene International Limited. All rights Reserved.