200,000+ products from a single source!
sales@angenechem.com
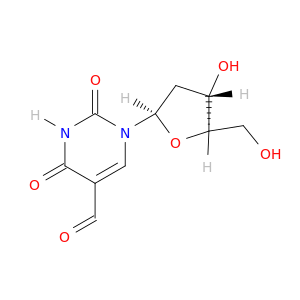
4494-26-2 | 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4-dioxopyrimidine-5-carbaldehyde
CAS No: 4494-26-2 Catalog No: AG003MRW MDL No:MFCD01689820
Product Description
Catalog Number:
AG003MRW
Chemical Name:
1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4-dioxopyrimidine-5-carbaldehyde
CAS Number:
4494-26-2
Molecular Formula:
C10H12N2O6
Molecular Weight:
256.2121
MDL Number:
MFCD01689820
IUPAC Name:
1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4-dioxopyrimidine-5-carbaldehyde
InChI:
InChI=1S/C10H12N2O6/c13-3-5-2-12(10(17)11-9(5)16)8-1-6(15)7(4-14)18-8/h2-3,6-8,14-15H,1,4H2,(H,11,16,17)/t6-,7+,8+/m0/s1
InChI Key:
MVORBLZUGBSUNB-XLPZGREQSA-N
SMILES:
OC[C@H]1O[C@H](C[C@@H]1O)n1cc(C=O)c(=O)[nH]c1=O
Properties
Complexity:
421
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
256.07g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
256.214g/mol
Monoisotopic Mass:
256.07g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
116A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.9
Literature
| Title | Journal |
|---|---|
| Design and synthesis of 3-carbamoylbenzoic acid derivatives as inhibitors of human apurinic/apyrimidinic endonuclease 1 (APE1). | ChemMedChem 20121001 |
| Efficient microwave-assisted synthesis, antibacterial activity and high fluorescence of 5 benzimidazolyl-2'-deoxyuridines. | Bioorganic & medicinal chemistry 20120101 |
| ATP chemosensitivity testing of new antitumor duplex drugs linking 3`-C-ethynylycytidine (ECyd) and 2´-deoxy-5-fluorouridine (5-FdU) in comparison to standard cytostatica and combinations thereof. | Investigational new drugs 20110601 |
| Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. | Analytical chemistry 20110315 |
| Selective detection of 5-formyl-2'-deoxyuridine, an oxidative lesion of thymidine, in DNA by a fluorogenic reagent. | Angewandte Chemie (International ed. in English) 20101102 |
| Solvent-free synthesis of pyrimidine nucleoside-aminophosphonate hybrids and their biological activity evaluation. | Nucleosides, nucleotides & nucleic acids 20100801 |
| Synthesis and properties of new fluorescent nucleosides and oligodeoxynucleotides derived from 5-formyl-2'-deoxyuridine. | Nucleic acids symposium series (2004) 20090101 |
| Synthesis of 5-formyl-2'-deoxyuridine and its incorporation into oligodeoxynucleotides. | Current protocols in nucleic acid chemistry 20081201 |
| Novel enamine derivatives of 5,6-dihydro-2'-deoxyuridine formed in reductive amination of 5-formyl-2'-deoxyuridine. | Nucleosides, nucleotides & nucleic acids 20080901 |
| Development of a novel selective detection method for 5-formyl-2'-deoxyuridine by fluorescent derivatization. | Nucleic acids symposium series (2004) 20080101 |
| Derivatization with Girard reagent T combined with LC-MS/MS for the sensitive detection of 5-formyl-2'-deoxyuridine in cellular DNA. | Analytical chemistry 20070101 |
| Structurally diverse 5-substituted pyrimidine nucleosides as inhibitors of Leishmania donovani promastigotes in vitro. | Bioorganic & medicinal chemistry letters 20061001 |
| 5-(Dimethoxymethyl)-2'-deoxyuridine: a novel gem diether nucleoside with anti-orthopoxvirus activity. | Journal of medicinal chemistry 20060601 |
| [Synthesis and antiviral activity of new 5-substituted 2'-deoxyuridine derivatives]. | Bioorganicheskaia khimiia 20050101 |
| Aggregation of RecA-derived peptides on single-stranded oligonucleotides triggered by schiff base-mediated crosslinking. | Bioorganic & medicinal chemistry letters 20030901 |
| Water mediated Dickerson-Drew-type crystal of DNA dodecamer containing 2'-deoxy-5-formyluridine. | Biophysical chemistry 20020328 |
| Mutations induced by 5-formyl-2'-deoxyuridine in Escherichia coli include base substitutions that can arise from mispairs of 5-formyluracil with guanine, cytosine and thymine. | Mutation research 20010509 |
| 5-Formyluracil and its nucleoside derivatives confer toxicity and mutagenicity to mammalian cells by interfering with normal RNA and DNA metabolism. | Toxicology letters 20010203 |
| Crystallization and preliminary X-ray analysis of a DNA dodecamer containing 2'-deoxy-5-formyluridine; what is the role of magnesium cation in crystallization of Dickerson-type DNA dodecamers? | Acta crystallographica. Section D, Biological crystallography 20010201 |
| Chemical cross-linking of peptides derived from RecA with single-stranded oligonucleotides containing 5-formyl-2'-deoxyuridine. | Nucleosides, nucleotides & nucleic acids 20010101 |
| Evidence for a Schiff base formation of peptides derived from RecA with single-stranded oligonucleotides containing 5-formyl-2'-deoxyuridine. | Nucleic acids research. Supplement (2001) 20010101 |
| X-ray analyses of DNA dodecamers containing 2'-deoxy-5-formyluridine. | Nucleic acids research. Supplement (2001) 20010101 |
| Antiherpes activity of (E)-5-(2-bromovinyl)- and 5-vinyl-1-beta-D-arabinofuranosyluracil and some other 5-substituted uracil arabinosyl nucleosides in two different cell lines. | Antiviral research 19830901 |
| Efficiency and selectivity of (E)-5-(2-bromovinyl)-2'-deoxyuridine and some other 5-substituted 2'-deoxypyrimidine nucleosides as anti-herpes agents. | Antiviral research 19820501 |
| Antiviral activity of 5-methylthiomethyl-2'-deoxyuridine and other 5-substituted 2'-deoxyuridines. | Biochemical pharmacology 19791115 |
Related Products
© 2019 Angene International Limited. All rights Reserved.