200,000+ products from a single source!
sales@angenechem.com
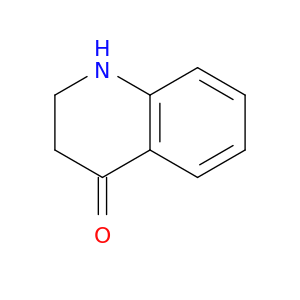
4295-36-7 | 2,3-Dihydro-1H-quinolin-4-one
CAS No: 4295-36-7 Catalog No: AG00BXI0 MDL No:MFCD00666394
Product Description
Catalog Number:
AG00BXI0
Chemical Name:
2,3-Dihydro-1H-quinolin-4-one
CAS Number:
4295-36-7
Molecular Formula:
C9H9NO
Molecular Weight:
147.1739
MDL Number:
MFCD00666394
IUPAC Name:
2,3-dihydro-1H-quinolin-4-one
InChI:
InChI=1S/C9H9NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-4,10H,5-6H2
InChI Key:
BUWPZNOVIHAWHW-UHFFFAOYSA-N
SMILES:
O=C1CCNc2c1cccc2
Properties
Complexity:
167
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
147.068g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
147.177g/mol
Monoisotopic Mass:
147.068g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
29.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.4
Literature
| Title | Journal |
|---|---|
| Formation of acridones by ethylene extrusion in the reaction of arynes with β-lactams and dihydroquinolinones. | The Journal of organic chemistry 20120720 |
| Stereoselective synthesis of fluorinated 2,3-dihydroquinolin-4(1H)-ones via a one-pot multistep transformation. | The Journal of organic chemistry 20120302 |
| Transition metal mediated construction of pyrrole ring on 2,3-dihydroquinolin-4(1H)-one: synthesis and pharmacological evaluation of novel tricyclic heteroarenes. | Organic & biomolecular chemistry 20110221 |
| Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. | BMC bioinformatics 20110101 |
| Asymmetric synthesis of 2-aryl-2,3-dihydro-4-quinolones via bifunctional thiourea-mediated intramolecular cyclization. | Organic letters 20101203 |
| Recent N-atom containing compounds from indo-pacific invertebrates. | Marine drugs 20100101 |
| Synthesis of 2,3-dihydroquinolin-4(1H)-ones through catalytic metathesis of o-alkynylanilines and aldehydes. | The Journal of organic chemistry 20090807 |
| 1-Benzyl-2,3-dihydro-quinolin-4(1H)-one. | Acta crystallographica. Section E, Structure reports online 20080201 |
| Efficient synthesis of 2-substituted 2,3-dihydro-4-quinolones as potential intermediates for 2-substituted 1,2,3,4-tetrahydro-4-quinolone antitumor agents. | Archives of pharmacal research 20060501 |
| Inhibition of clinical isolates of human cytomegalovirus and varicella zoster virus by PNU-183792, a 4-oxo-dihydroquinoline. | Journal of medical virology 20021001 |
| Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. | Antiviral research 20020401 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.