200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 4143-64-0
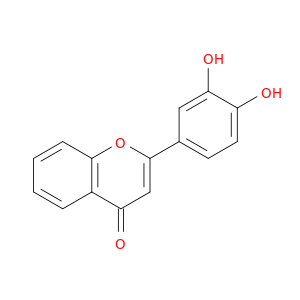
4143-64-0 | 2-(3,4-Dihydroxyphenyl)-4H-chromen-4-one
CAS No: 4143-64-0 Catalog No: AG003IHR MDL No:MFCD00017600
Product Description
Catalog Number:
AG003IHR
Chemical Name:
2-(3,4-Dihydroxyphenyl)-4H-chromen-4-one
CAS Number:
4143-64-0
Molecular Formula:
C15H10O4
Molecular Weight:
254.2375
MDL Number:
MFCD00017600
IUPAC Name:
2-(3,4-dihydroxyphenyl)chromen-4-one
InChI:
InChI=1S/C15H10O4/c16-11-6-5-9(7-13(11)18)15-8-12(17)10-3-1-2-4-14(10)19-15/h1-8,16,18H
InChI Key:
SRNPMQHYWVKBAV-UHFFFAOYSA-N
SMILES:
Oc1ccc(cc1O)c1cc(=O)c2c(o1)cccc2
UNII:
KOH101S66V
Properties
Complexity:
390
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
254.058g/mol
Formal Charge:
0
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
254.241g/mol
Monoisotopic Mass:
254.058g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
66.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. | Chemico-biological interactions 20151205 |
| The O-methylation of chrysin markedly improves its intestinal anti-inflammatory properties: Structure-activity relationships of flavones. | Biochemical pharmacology 20131215 |
| Inhibition of platelet-mediated arterial thrombosis and platelet granule exocytosis by 3',4'-dihydroxyflavonol and quercetin. | Platelets 20130101 |
| Discovery of diverse human dihydroorotate dehydrogenase inhibitors as immunosuppressive agents by structure-based virtual screening. | Journal of medicinal chemistry 20121011 |
| 3,3',4',5,5'-Pentahydroxyflavone is a potent inhibitor of amyloid β fibril formation. | Neuroscience letters 20120328 |
| Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. | Bioorganic & medicinal chemistry 20110501 |
| Characterization of an O-methyltransferase from Streptomyces avermitilis MA-4680. | Journal of microbiology and biotechnology 20100901 |
| 7-Hydroxy-benzopyran-4-one derivatives: a novel pharmacophore of peroxisome proliferator-activated receptor alpha and -gamma (PPARalpha and gamma) dual agonists. | Journal of medicinal chemistry 20091112 |
| Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. | Nutrition and cancer 20090101 |
| Iron-binding properties of plant phenolics and cranberry's bio-effects. | Dalton transactions (Cambridge, England : 2003) 20071121 |
| 3-Hydroxychromones as cyclin-dependent kinase inhibitors: synthesis and biological evaluation. | Bioorganic & medicinal chemistry letters 20070301 |
| Evaluation of novel chromogenic substrates for the detection of bacterial beta-glucosidase. | Journal of applied microbiology 20070201 |
| The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. | Biological & pharmaceutical bulletin 20070101 |
| Spectroscopic and theoretical studies of the Zn(II) chelation with hydroxyflavones. | The journal of physical chemistry. A 20061116 |
| Influence of biotransformation of luteolin, luteolin 7-O-glucoside, 3',4'-dihydroxyflavone and apigenin by cultured rat hepatocytes on antioxidative capacity and inhibition of EGF receptor tyrosine kinase activity. | Planta medica 20060601 |
| Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. | The Journal of biological chemistry 20050909 |
| Time dependent density functional theory study of electronic absorption properties of lead(II) complexes with a series of hydroxyflavones. | The journal of physical chemistry. A 20050804 |
| Stability of ferric complexes with 3-hydroxyflavone (flavonol), 5,7-dihydroxyflavone (chrysin), and 3',4'-dihydroxyflavone. | Journal of agricultural and food chemistry 20050420 |
| Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. | Biochemical pharmacology 20050215 |
| Catecholic flavonoids acting as telomerase inhibitors. | Journal of medicinal chemistry 20041216 |
| 3',4'-Dihydroxyflavonol reduces infarct size and injury associated with myocardial ischaemia and reperfusion in sheep. | British journal of pharmacology 20040601 |
| The regioselectivity of glutathione adduct formation with flavonoid quinone/quinone methides is pH-dependent. | Chemical research in toxicology 20020301 |
| Complexes of Al(III) with 3'4'-dihydroxy-flavone: characterization, theoretical and spectroscopic study. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20010301 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.