200,000+ products from a single source!
sales@angenechem.com
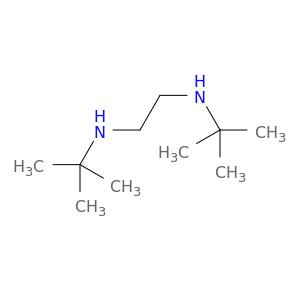
4062-60-6 | N,N'-Di-tert-butylethylenediamine
CAS No: 4062-60-6 Catalog No: AG0037IC MDL No:MFCD00014996
Product Description
Catalog Number:
AG0037IC
Chemical Name:
N,N'-Di-tert-butylethylenediamine
CAS Number:
4062-60-6
Molecular Formula:
C10H24N2
Molecular Weight:
172.3110
MDL Number:
MFCD00014996
IUPAC Name:
N,N'-ditert-butylethane-1,2-diamine
InChI:
InChI=1S/C10H24N2/c1-9(2,3)11-7-8-12-10(4,5)6/h11-12H,7-8H2,1-6H3
InChI Key:
KGHYGBGIWLNFAV-UHFFFAOYSA-N
SMILES:
CC(NCCNC(C)(C)C)(C)C
EC Number:
223-769-6
UNII:
6WAI8U5V0W
Properties
Complexity:
101
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
172.194g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
172.316g/mol
Monoisotopic Mass:
172.194g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
24.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.3
Literature
| Title | Journal |
|---|---|
| Reaction coordinate of a functional model of tyrosinase: spectroscopic and computational characterization. | Journal of the American Chemical Society 20090513 |
| Theoretical study of the hydroxylation of phenolates by the Cu(2)O (2)(N,N'-dimethylethylenediamine) (2) (2+) complex. | Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry 20090201 |
| Syntheses, structures, luminescence and electrochemistry of benzene- and biphenyl-centered bis- and tris-1,3,2-diazaboroles and -1,3,2-diazaborolidines. | Dalton transactions (Cambridge, England : 2003) 20060507 |
| Stable optically pure phosphino(silyl)carbenes: reagents for highly enantioselective cyclopropanation reactions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20040419 |
| A stabilized mu-eta(2):eta(2) peroxodicopper(II) complex with a secondary diamine ligand and its tyrosinase-like reactivity. | Journal of the American Chemical Society 20020814 |
| Structure-activity studies leading to ethambutol, a new type of antituberculous compound. | Annals of the New York Academy of Sciences 19660420 |
Related Products
© 2019 Angene International Limited. All rights Reserved.