200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 4046-02-0
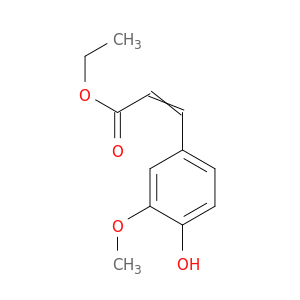
4046-02-0 | Ethyl 3-(4-hydroxy-3-methoxyphenyl)acrylate
CAS No: 4046-02-0 Catalog No: AG003LHZ MDL No:MFCD00009190
Product Description
Catalog Number:
AG003LHZ
Chemical Name:
Ethyl 3-(4-hydroxy-3-methoxyphenyl)acrylate
CAS Number:
4046-02-0
Molecular Formula:
C12H14O4
Molecular Weight:
222.2372
MDL Number:
MFCD00009190
IUPAC Name:
ethyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate
InChI:
InChI=1S/C12H14O4/c1-3-16-12(14)7-5-9-4-6-10(13)11(8-9)15-2/h4-8,13H,3H2,1-2H3/b7-5+
InChI Key:
ATJVZXXHKSYELS-FNORWQNLSA-N
SMILES:
CCOC(=O)C=Cc1ccc(c(c1)OC)O
EC Number:
223-745-5
UNII:
5B8915UELW
NSC Number:
14879
Properties
Complexity:
249
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
222.089g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
222.24g/mol
Monoisotopic Mass:
222.089g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
55.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Biological activity evaluation and structure-activity relationships analysis of ferulic acid and caffeic acid derivatives for anticancer. | Bioorganic & medicinal chemistry letters 20121001 |
| Hydroxycinnamic acid ethyl esters as precursors to ethylphenols in wine. | Journal of agricultural and food chemistry 20120307 |
| Taste-guided fractionation and instrumental analysis of hydrophobic compounds in sake. | Bioscience, biotechnology, and biochemistry 20120101 |
| Synthesis of ethyl ferulate in organic medium using celite-immobilized lipase. | Bioresource technology 20110201 |
| Identification of curcumin derivatives as human glyoxalase I inhibitors: A combination of biological evaluation, molecular docking, 3D-QSAR and molecular dynamics simulation studies. | Bioorganic & medicinal chemistry 20110201 |
| Two new cytotoxic labdane diterpenes from the rhizomes of Hedychium coronarium. | Bioorganic & medicinal chemistry letters 20101215 |
| Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders. | Advances in experimental medicine and biology 20100101 |
| 1,3-Diferuloyl-sn-glycerol from the biocatalytic transesterification of ethyl 4-hydroxy-3-methoxy cinnamic acid (ethyl ferulate) and soybean oil. | Biotechnology letters 20090601 |
| Dual response surface-optimized process for feruloylated diacylglycerols by selective lipase-catalyzed transesterification in solvent free system. | Bioresource technology 20090601 |
| New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. | European journal of medicinal chemistry 20090501 |
| Ferulate-coniferyl alcohol cross-coupled products formed by radical coupling reactions. | Planta 20090401 |
| Protective effect of ferulic acid ethyl ester against oxidative stress mediated by UVB irradiation in human epidermal melanocytes. | Free radical research 20090401 |
| Peroxidase-catalyzed oligomerization of ferulic acid esters. | Journal of agricultural and food chemistry 20081112 |
| Caffeic acid phenethyl ester and its related compounds limit the functional alterations of the isolated mouse brain and liver mitochondria submitted to in vitro anoxia-reoxygenation: relationship to their antioxidant activities. | Biochimica et biophysica acta 20080401 |
| Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. | Clinics in dermatology 20080101 |
| A novel recombinant ethyl ferulate esterase from Burkholderia multivorans. | Journal of applied microbiology 20071101 |
| Screening and analysis of an antineoplastic compound in Rhizoma Chuanxiong by means of in vitro metabolism and HPLC-MS. | Analytical and bioanalytical chemistry 20060901 |
| In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. | Journal of neuroscience research 20060801 |
| Enzymatic synthesis of cinnamic acid derivatives. | Biotechnology letters 20060401 |
| In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. | Neurochemistry international 20060301 |
| Characterization of enzymatically synthesized diferulate. | Annals of the New York Academy of Sciences 20050601 |
| Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. | Journal of neurochemistry 20050201 |
| Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. | Antioxidants & redox signaling 20041001 |
| Protective effects of the lipophilic redox conjugate tocopheryl succinyl-ethyl ferulate on HIV replication. | FEBS letters 19971124 |
Related Products
© 2019 Angene International Limited. All rights Reserved.