200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 3848-24-6
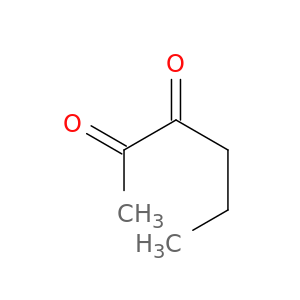
3848-24-6 | 2,3-HEXANEDIONE
CAS No: 3848-24-6 Catalog No: AG003FKX MDL No:MFCD00009398
Product Description
Catalog Number:
AG003FKX
Chemical Name:
2,3-HEXANEDIONE
CAS Number:
3848-24-6
Molecular Formula:
C6H10O2
Molecular Weight:
114.1424
MDL Number:
MFCD00009398
IUPAC Name:
hexane-2,3-dione
InChI:
InChI=1S/C6H10O2/c1-3-4-6(8)5(2)7/h3-4H2,1-2H3
InChI Key:
MWVFCEVNXHTDNF-UHFFFAOYSA-N
SMILES:
CCCC(=O)C(=O)C
EC Number:
223-350-8
UNII:
559ANR3NVS
NSC Number:
31665
FEMA Number:
2558
UN Number:
1224
Properties
Complexity:
105
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
114.068g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
114.144g/mol
Monoisotopic Mass:
114.068g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
34.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.4
Literature
| Title | Journal |
|---|---|
| Curcumin is a tight-binding inhibitor of the most efficient human daunorubicin reductase--Carbonyl reductase 1. | Chemico-biological interactions 20150605 |
| Biocatalytic production of alpha-hydroxy ketones and vicinal diols by yeast and human aldo-keto reductases. | Chemico-biological interactions 20130225 |
| S-nitrosoglutathione covalently modifies cysteine residues of human carbonyl reductase 1 and affects its activity. | Chemico-biological interactions 20130225 |
| Rapid and sensitive determination of the intermediates of advanced glycation end products in the human nail by ultra-performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. | Analytical biochemistry 20120515 |
| Bioinformatic and biochemical characterization of DCXR and DHRS2/4 from Caenorhabditis elegans. | Chemico-biological interactions 20110530 |
| Identification and measurement of diacetyl substitutes in dry bakery mix production. | Journal of occupational and environmental hygiene 20110201 |
| (R)-3-hydroxyhexan-2-one is a major pheromone component of Anelaphus inflaticollis (Coleoptera: Cerambycidae). | Environmental entomology 20091001 |
| Characterization of a rat NADPH-dependent aldo-keto reductase (AKR1B13) induced by oxidative stress. | Chemico-biological interactions 20090316 |
| Probing mechanisms of axonopathy. Part II: Protein targets of 2,5-hexanedione, the neurotoxic metabolite of the aliphatic solvent n-hexane. | Toxicological sciences : an official journal of the Society of Toxicology 20090201 |
| Bis(guanylhydrazones) negatively affect in vitro germination of kiwifruit pollen and alter the endogenous polyamine pool. | Plant biology (Stuttgart, Germany) 20080501 |
| A comparison of the apoptotic and cytotoxic effects of hexanedione derivatives on human non-neuronal lines and the neuroblastoma line SH-SY5Y. | Basic & clinical pharmacology & toxicology 20080101 |
| Characterization of an oligomeric carbonyl reductase of dog liver: its identity with peroxisomal tetrameric carbonyl reductase. | Biological & pharmaceutical bulletin 20070901 |
| Mechanistic diversity in the RuBisCO superfamily: the 'enolase' in the methionine salvage pathway in Geobacillus kaustophilus. | Biochemistry 20070403 |
| Apoptotic and necrotic effects of hexanedione derivatives on the human neuroblastoma line SK-N-SH. | Toxicology 20070307 |
| Mass, IR, electronic and EPR spectral studies on transition metal complexes with a new tetradentate 12-membered new macrocyclic ligand. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20041101 |
| Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. | Journal of chemical ecology 20040801 |
| The kinetic method as a structural diagnostic tool: ionized alpha-diketones as loosely one-electron bonded diacylium ion dimers. | European journal of mass spectrometry (Chichester, England) 20030101 |
| Stereoselective oxidation of aliphatic diols and reduction of hydroxy-ketones with galactitol dehydrogenase from Rhodobacter sphaeroides D. | Communications in agricultural and applied biological sciences 20030101 |
| Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. | The Biochemical journal 19991015 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.