200,000+ products from a single source!
sales@angenechem.com
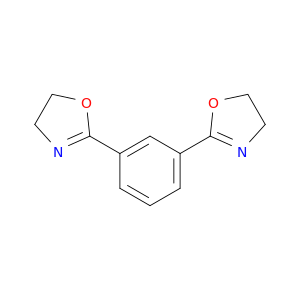
34052-90-9 | 1,3-Bis(4,5-dihydrooxazol-2-yl)benzene
CAS No: 34052-90-9 Catalog No: AG003DHL MDL No:MFCD00191606
Product Description
Catalog Number:
AG003DHL
Chemical Name:
1,3-Bis(4,5-dihydrooxazol-2-yl)benzene
CAS Number:
34052-90-9
Molecular Formula:
C12H12N2O2
Molecular Weight:
216.2359
MDL Number:
MFCD00191606
IUPAC Name:
2-[3-(4,5-dihydro-1,3-oxazol-2-yl)phenyl]-4,5-dihydro-1,3-oxazole
InChI:
InChI=1S/C12H12N2O2/c1-2-9(11-13-4-6-15-11)8-10(3-1)12-14-5-7-16-12/h1-3,8H,4-7H2
InChI Key:
HMOZDINWBHMBSQ-UHFFFAOYSA-N
SMILES:
C1CN=C(O1)c1cccc(c1)C1=NCCO1
EC Number:
421-510-3
Properties
Complexity:
295
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
216.09g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
216.24g/mol
Monoisotopic Mass:
216.09g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
43.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| Tetra-kis[μ-1,3-bis-(4,5-dihydro-1,3-oxazol-2-yl)benzene-κ(2)N:N']tris-ilver(I) tris-(hexa-fluoridophosphate). | Acta crystallographica. Section E, Structure reports online 20120901 |
| Synthesis of poly(2-oxazoline)-based hydrogels with tailor-made swelling degrees capable of stimuli-triggered compound release. | Macromolecular rapid communications 20111115 |
| Bis(oxazolinyl)phenyl transition metal complexes: synthesis, asymmetric catalysis, and coordination chemistry. | Chemical record (New York, N.Y.) 20070101 |
| Asymmetric conjugate reduction of alpha,beta-unsaturated ketones and esters with chiral rhodium(2,6-bisoxazolinylphenyl) catalysts. | Chemistry (Weinheim an der Bergstrasse, Germany) 20051216 |
Related Products
© 2019 Angene International Limited. All rights Reserved.