200,000+ products from a single source!
sales@angenechem.com
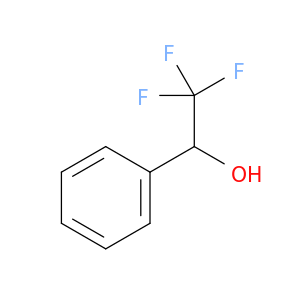
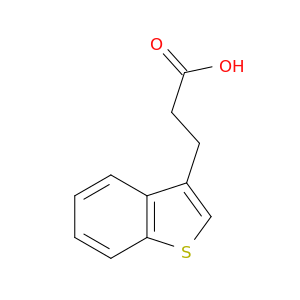
340-05-6 | 2,2,2-Trifluoro-1-phenylethanol
CAS No: 340-05-6 Catalog No: AG003ELY MDL No:MFCD00004498
Product Description
Catalog Number:
AG003ELY
Chemical Name:
2,2,2-Trifluoro-1-phenylethanol
CAS Number:
340-05-6
Molecular Formula:
C8H7F3O
Molecular Weight:
176.1358
MDL Number:
MFCD00004498
IUPAC Name:
2,2,2-trifluoro-1-phenylethanol
InChI:
InChI=1S/C8H7F3O/c9-8(10,11)7(12)6-4-2-1-3-5-6/h1-5,7,12H
InChI Key:
VNOMEAQPOMDWSR-UHFFFAOYSA-N
SMILES:
OC(C(F)(F)F)c1ccccc1
EC Number:
206-429-1
NSC Number:
20214
Properties
Complexity:
138
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
176.045g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
176.138g/mol
Monoisotopic Mass:
176.045g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
20.2A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Chiral recognition in metal-organic frameworks studied by solid-state NMR spectroscopy using chiral solvating agents. | Chemical communications (Cambridge, England) 20121104 |
| Characterization and further stabilization of a new anti-prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds. | Bioresource technology 20120101 |
| Enantioselective HF loss promoted by resonant two-photon ionization of supersonically expanded (R)-1-phenyl-2,2,2-trifluoroethanol clusters. | The journal of physical chemistry. A 20091231 |
| NMR and molecular modeling of the dimeric self-association of the enantiomers of 1,1'-bi-2-naphthol and 1-phenyl-2,2,2-trifluoroethanol in the solution state and their relevance to enantiomer self-disproportionation on achiral-phase chromatography (ESDAC). | Organic & biomolecular chemistry 20090207 |
| Monosolvation of R-1-phenyl-2,2,2-trifluoroethanol with amines: configurational effects on the excitation, ionization, and fragmentation of diastereomeric complexes. | The journal of physical chemistry. A 20071213 |
| Substituent effects on the stereochemistry of gas-phase intracomplex nucleophilic substitutions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20061016 |
| Unveiling the 'booster effect' of fluorinated alcohol solvents: aggregation-induced conformational changes and cooperatively enhanced H-bonding. | Journal of the American Chemical Society 20060705 |
| Synthesis and photodynamic activity of zinc(II) phthalocyanine derivatives bearing methoxy and trifluoromethylbenzyloxy substituents in homogeneous and biological media. | Bioorganic & medicinal chemistry 20050103 |
Related Products
© 2019 Angene International Limited. All rights Reserved.