200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 3149-28-8
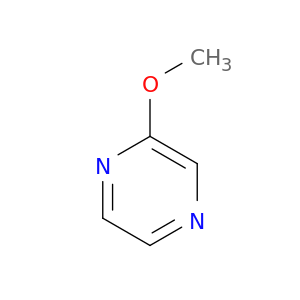
3149-28-8 | 2-Methoxypyrazine
CAS No: 3149-28-8 Catalog No: AG003HKJ MDL No:MFCD00006126
Product Description
Catalog Number:
AG003HKJ
Chemical Name:
2-Methoxypyrazine
CAS Number:
3149-28-8
Molecular Formula:
C5H6N2O
Molecular Weight:
110.1139
MDL Number:
MFCD00006126
IUPAC Name:
2-methoxypyrazine
InChI:
InChI=1S/C5H6N2O/c1-8-5-4-6-2-3-7-5/h2-4H,1H3
InChI Key:
WKSXRWSOSLGSTN-UHFFFAOYSA-N
SMILES:
COc1cnccn1
EC Number:
221-579-8
UNII:
RYD35T7F4T
FEMA Number:
3302
Properties
Complexity:
67.4
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
110.048g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
110.116g/mol
Monoisotopic Mass:
110.048g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
35A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.7
Literature
| Title | Journal |
|---|---|
| Classification of grape berries according to diameter and total soluble solids to study the effect of light and temperature on methoxypyrazine, glutathione, and hydroxycinnamate evolution during ripening of Sauvignon blanc (Vitis vinifera L.). | Journal of agricultural and food chemistry 20120919 |
| Survey of 3-alkyl-2-methoxypyrazine content of South African Sauvignon blanc wines using a novel LC-APCI-MS/MS method. | Journal of agricultural and food chemistry 20091028 |
| Aroma composition of red wines by different extraction methods and Gas Chromatography-SIM/MASS spectrometry analysis. | Annali di chimica 20050601 |
| How low pH can intensify beta-damascenone and dimethyl trisulfide production through beer aging. | Journal of agricultural and food chemistry 20020925 |
| S-Adenosyl-L-methionine-dependent O-methylation of 2-hydroxy-3-alkylpyrazine in wine grapes: a putative final step of methoxypyrazine biosynthesis. | Bioscience, biotechnology, and biochemistry 20010401 |
| Structure-activity relationship of ligands of uracil phosphoribosyltransferase from Toxoplasma gondii. | Biochemical pharmacology 19940817 |
Related Products
© 2019 Angene International Limited. All rights Reserved.