200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 30358-74-8
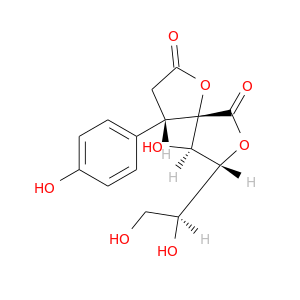
30358-74-8 | 1,7-Dioxaspiro[4.4]nonane-2,6-dione, 8-[(1S)-1,2-dihydroxyethyl]-9-hydroxy-4-(4-hydroxyphenyl)-, (4S,5S,8R,9R)- (9CI)
CAS No: 30358-74-8 Catalog No: AG002ZU6 MDL No:
Product Description
Catalog Number:
AG002ZU6
Chemical Name:
1,7-Dioxaspiro[4.4]nonane-2,6-dione, 8-[(1S)-1,2-dihydroxyethyl]-9-hydroxy-4-(4-hydroxyphenyl)-, (4S,5S,8R,9R)- (9CI)
CAS Number:
30358-74-8
Molecular Formula:
C15H16O8
Molecular Weight:
324.2827
IUPAC Name:
(4S,8R,9R)-8-[(1S)-1,2-dihydroxyethyl]-9-hydroxy-4-(4-hydroxyphenyl)-1,7-dioxaspiro[4.4]nonane-2,6-dione
InChI:
InChI=1S/C15H16O8/c16-6-10(18)12-13(20)15(14(21)22-12)9(5-11(19)23-15)7-1-3-8(17)4-2-7/h1-4,9-10,12-13,16-18,20H,5-6H2/t9-,10-,12+,13+,15?/m0/s1
InChI Key:
JVCLQSJXGOABTC-IPNWIPTBSA-N
SMILES:
OC[C@@H]([C@H]1OC(=O)[C@]2([C@@H]1O)OC(=O)C[C@H]2c1ccc(cc1)O)O
Properties
Complexity:
486
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
324.085g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
324.285g/mol
Monoisotopic Mass:
324.085g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
134A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.5
Literature
| Title | Journal |
|---|---|
| Anti-tuberculosis neolignans from Piper regnellii. | Phytomedicine : international journal of phytotherapy and phytopharmacology 20130515 |
| Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. | Journal of natural products 20110826 |
| Antinociceptive properties of conocarpan and orientin obtained from Piper solmsianum C. DC. var. solmsianum (Piperaceae). | Journal of natural medicines 20101001 |
| Antifungal activities of compounds isolated from Piper abutiloides Kunth. | Mycoses 20091101 |
| Asymmetric synthesis of neolignans (-)-epi-conocarpan and (+)-conocarpan via Rh(II)-catalyzed C-H insertion process and revision of the absolute configuration of (-)-epi-conocarpan. | The Journal of organic chemistry 20090605 |
| Synthesis of (-)-conocarpan by two routes based on radical cyclization and establishment of its absolute configuration. | Organic & biomolecular chemistry 20080521 |
| Total synthesis of (-)-conocarpan and assignment of the absolute configuration by chemical methods. | Chemical communications (Cambridge, England) 20070607 |
| Antioxidant and photoprotective activity of a lipophilic extract containing neolignans from Krameria triandra roots. | Planta medica 20020301 |
| Antifungal principles from Piper fulvescens. | Planta medica 20011201 |
| Krametosan, a new trinorlignan from the roots of Krameria tomentosa. | Natural product letters 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







