200,000+ products from a single source!
sales@angenechem.com
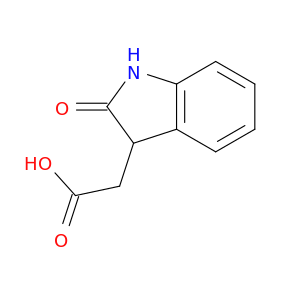
2971-31-5 | 1H-Indole-3-acetic acid, 2,3-dihydro-2-oxo-
CAS No: 2971-31-5 Catalog No: AG002Z6E MDL No:MFCD09035909
Product Description
Catalog Number:
AG002Z6E
Chemical Name:
1H-Indole-3-acetic acid, 2,3-dihydro-2-oxo-
CAS Number:
2971-31-5
Molecular Formula:
C10H9NO3
Molecular Weight:
191.1834
MDL Number:
MFCD09035909
IUPAC Name:
2-(2-oxo-1,3-dihydroindol-3-yl)acetic acid
InChI:
InChI=1S/C10H9NO3/c12-9(13)5-7-6-3-1-2-4-8(6)11-10(7)14/h1-4,7H,5H2,(H,11,14)(H,12,13)
InChI Key:
ILGMGHZPXRDCCS-UHFFFAOYSA-N
SMILES:
OC(=O)CC1C(=O)Nc2c1cccc2
Properties
Complexity:
264
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
191.058g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
191.186g/mol
Monoisotopic Mass:
191.058g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
66.4A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
0.3
Literature
| Title | Journal |
|---|---|
| Extracts and constituents of Rubus chingii with 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. | International journal of molecular sciences 20110101 |
| Metabolomic, transcriptional, hormonal, and signaling cross-talk in superroot2. | Molecular plant 20100101 |
| Antioxidative activities of Oxindole-3-acetic acid derivatives from supersweet corn powder. | Bioscience, biotechnology, and biochemistry 20100101 |
| Quantitative analysis of indole-3-acetic acid metabolites in Arabidopsis. | Plant physiology 20011201 |
| Metabolism of indole-3-acetic acid by orange (Citrus sinensis) flavedo tissue during fruit development. | Phytochemistry 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.