200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 2922-40-9
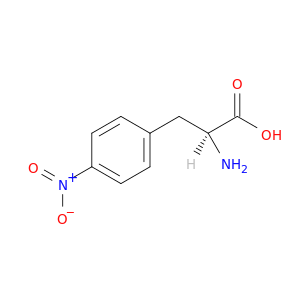
2922-40-9 | Phenylalanine, 4-nitro-
CAS No: 2922-40-9 Catalog No: AG002XRR MDL No:MFCD00007384
Product Description
Catalog Number:
AG002XRR
Chemical Name:
Phenylalanine, 4-nitro-
CAS Number:
2922-40-9
Molecular Formula:
C9H10N2O4
Molecular Weight:
210.1867
MDL Number:
MFCD00007384
IUPAC Name:
2-amino-3-(4-nitrophenyl)propanoic acid
InChI:
InChI=1S/C9H10N2O4/c10-8(9(12)13)5-6-1-3-7(4-2-6)11(14)15/h1-4,8H,5,10H2,(H,12,13)
InChI Key:
GTVVZTAFGPQSPC-UHFFFAOYSA-N
SMILES:
OC(=O)[C@H](Cc1ccc(cc1)[N+](=O)[O-])N
EC Number:
220-868-6
Properties
Complexity:
243
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
210.064g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
210.189g/mol
Monoisotopic Mass:
210.064g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
109A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.2
Literature
| Title | Journal |
|---|---|
| Comparison of fluorescence reagents for simultaneous determination of hydroxylated phenylalanine and nitrated tyrosine by high-performance liquid chromatography with fluorescence detection. | Biomedical chromatography : BMC 20120101 |
| Nitro-phenylalanine: a novel sensor for heat transfer in peptides. | The journal of physical chemistry. A 20110324 |
| Probing local environments with the infrared probe: L-4-nitrophenylalanine. | The journal of physical chemistry. B 20110317 |
| Genetic incorporation of unnatural amino acids into proteins in Mycobacterium tuberculosis. | PloS one 20100101 |
| 1H NMR determination of beta-N-methylamino-L-alanine (L-BMAA) in environmental and biological samples. | Toxicon : official journal of the International Society on Toxinology 20090401 |
| 2-Ammonio-3-(4-nitro-phen-yl)propanoate monohydrate. | Acta crystallographica. Section E, Structure reports online 20080801 |
| (S)-2-Ammonio-3-(4-nitro-phen-yl)propanoate monohydrate. | Acta crystallographica. Section E, Structure reports online 20080601 |
| Profiling of proteolytic enzymes in the gut of the tick Ixodes ricinus reveals an evolutionarily conserved network of aspartic and cysteine peptidases. | Parasites & vectors 20080101 |
| The genetic incorporation of a distance probe into proteins in Escherichia coli. | Journal of the American Chemical Society 20060412 |
| Efficient incorporation of a nonnatural amino acid into a protein in an insect cell-free translation system. | Nucleic acids symposium series (2004) 20060101 |
| Neighbouring group processes in the deamination of protonated phenylalanine derivatives. | Organic & biomolecular chemistry 20051021 |
| Synthesis of new bivalent peptides for applications in the Affinity Enhancement System. | Bioconjugate chemistry 20050101 |
| A novel competitive ELISA for both free and protein-bound nitrotyrosine. | Hybridoma and hybridomics 20031201 |
| Position-specific incorporation of a fluorophore-quencher pair into a single streptavidin through orthogonal four-base codon/anticodon pairs. | Journal of the American Chemical Society 20021211 |
| Influence of a ring substituent on the tendency to form H(2)O adducts to Ag(+) complexes with phenylalanine analogues in an ion trap mass spectrometer. | Journal of mass spectrometry : JMS 20020401 |
| 6-hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. | Journal of neurochemistry 20010801 |
| Increase in fluorescence upon the hydrolysis of tyrosine peptides: application to proteinase assays. | Analytical biochemistry 19950501 |
| Sensitive, soluble chromogenic substrates for HIV-1 proteinase. | The Journal of biological chemistry 19900515 |
| Comparison of mu-, delta- and kappa-receptor binding sites through pharmacologic evaluation of p-nitrophenylalanine analogs of opioid peptides. | Life sciences 19830101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.