200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 29191-53-5
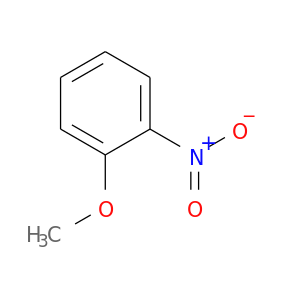
29191-53-5 | Benzene, methoxynitro-
CAS No: 29191-53-5 Catalog No: AG002XMV MDL No:
Product Description
Catalog Number:
AG002XMV
Chemical Name:
Benzene, methoxynitro-
CAS Number:
29191-53-5
Molecular Formula:
C7H7NO3
Molecular Weight:
153.1354
IUPAC Name:
1-methoxy-2-nitrobenzene
InChI:
InChI=1S/C7H7NO3/c1-11-7-5-3-2-4-6(7)8(9)10/h2-5H,1H3
InChI Key:
CFBYEGUGFPZCNF-UHFFFAOYSA-N
SMILES:
COc1ccccc1[N+](=O)[O-]
EC Number:
202-052-1
UNII:
ZRE7HLZ17K
NSC Number:
5506
RTECS Number:
BZ8790000
UN Number:
2730
Properties
Complexity:
143
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
153.043g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
153.137g/mol
Monoisotopic Mass:
153.043g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
55A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Investigation of the early-response genes in chemical-induced renal carcinogenicity for the prediction of chemical carcinogenicity in rats. | The Journal of toxicological sciences 20170101 |
| Early Detection of Genotoxic Urinary Bladder Carcinogens by Immunohistochemistry for γ-H2AX. | Toxicological sciences : an official journal of the Society of Toxicology 20151201 |
| Human cytochrome-P450 enzymes metabolize N-(2-methoxyphenyl)hydroxylamine, a metabolite of the carcinogens o-anisidine and o-nitroanisole, thereby dictating its genotoxicity. | Mutation research 20111224 |
| FT-IR and FT-Raman spectroscopic investigation, computed vibrational frequency analysis and IR intensity and Raman activity peak resemblance analysis on 2-nitroanisole using HF and DFT (B3LYP and B3PW91) calculations. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20111201 |
| Preferential solvation in alkan-1-ol/alkylbenzoate binary mixtures by solvatochromic probes. | The journal of physical chemistry. B 20110901 |
| o-Nitroanisole. | Report on carcinogens : carcinogen profiles 20110101 |
| Identification of rat cytochromes P450 metabolizing N-(2-methoxyphenyl)hydroxylamine, a human metabolite of the environmental pollutants and carcinogens o-anisidine and o-nitroanisole. | Neuro endocrinology letters 20100101 |
| Genotoxic mechanisms for the carcinogenicity of the environmental pollutants and carcinogens o-anisidine and 2-nitroanisole follow from adducts generated by their metabolite N-(2-methoxyphenyl)-hydroxylamine with deoxyguanosine in DNA. | Interdisciplinary toxicology 20090301 |
| Rat cytochromes P450 oxidize 2-nitrophenol, a human metabolite of carcinogenic 2-nitroanisole. | Neuro endocrinology letters 20090101 |
| Cytochrome P450-mediated metabolism of N-(2-methoxyphenyl)-hydroxylamine, a human metabolite of the environmental pollutants and carcinogens o-anisidine and o-nitroanisole. | Interdisciplinary toxicology 20081201 |
| Oxidation of carcinogenic 2-nitroanisole by rat cytochromes P450 - similarity between human and rat enzymes. | Interdisciplinary toxicology 20080901 |
| Structure and performance of silica-based monolithic HPLC columns. | Journal of separation science 20080801 |
| In vivo Comet assay on isolated kidney cells to distinguish genotoxic carcinogens from epigenetic carcinogens or cytotoxic compounds. | Mutation research 20070615 |
| Oxidative detoxication of carcinogenic 2-nitroanisole by human, rat and rabbit cytochrome P450. | Neuro endocrinology letters 20061201 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Tumorigenic potential and the molecular mechanism of the carcinogenic effect exerted by 2-nitroanisole. | Anticancer research 20060101 |
| Carcinogenic pollutants o-nitroanisole and o-anisidine are substrates and inducers of cytochromes P450. | Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 20051201 |
| Identification of a genotoxic mechanism for 2-nitroanisole carcinogenicity and of its carcinogenic potential for humans. | Carcinogenesis 20040501 |
| Enzymes involved in the metabolism of the carcinogen 2-nitroanisole: evidence for its oxidative detoxication by human cytochromes P450. | Chemical research in toxicology 20040501 |
| Association of liver hemangiosarcoma and secondary iron overload in B6C3F1 mice--the National Toxicology Program experience. | Toxicologic pathology 20040101 |
| o-Nitroanisole. | Report on carcinogens : carcinogen profiles 20040101 |
| 3-pyrrolines are mechanism-based inactivators of the quinone-dependent amine oxidases but only substrates of the flavin-dependent amine oxidases. | Journal of the American Chemical Society 20021016 |
| o-Nitroanisole. | Report on carcinogens : carcinogen profiles 20020101 |
| Transcriptional activation of stress genes and cytotoxicity in human liver carcinoma cells (HepG2) exposed to 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and 2,6-dinitrotoluene. | Environmental toxicology 20010601 |
| Synthesis and antibacterial activity of 5-nitrofuryl and 3-methoxy-2-nitrophenyl derivatives of 6 beta-aminopenicillanic, 7 beta-aminocephalosporanic and 7 beta-aminodesacetoxy-cephalosporanic acids. | Arzneimittel-Forschung 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.