200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 291-21-4
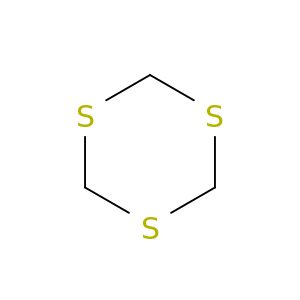
291-21-4 | 1,3,5-Trithiane
CAS No: 291-21-4 Catalog No: AG002XBC MDL No:MFCD00006653
Product Description
Catalog Number:
AG002XBC
Chemical Name:
1,3,5-Trithiane
CAS Number:
291-21-4
Molecular Formula:
C3H6S3
Molecular Weight:
138.2747
MDL Number:
MFCD00006653
IUPAC Name:
1,3,5-trithiane
InChI:
InChI=1S/C3H6S3/c1-4-2-6-3-5-1/h1-3H2
InChI Key:
LORRLQMLLQLPSJ-UHFFFAOYSA-N
SMILES:
S1CSCSC1
EC Number:
206-029-7
NSC Number:
1937
Properties
Complexity:
21.5
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
137.963g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
138.261g/mol
Monoisotopic Mass:
137.963g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
75.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Use of ESI-MS to determine reaction pathway for hydrogen sulphide scavenging with 1,3,5-tri-(2-hydroxyethyl)-hexahydro-s-triazine. | European journal of mass spectrometry (Chichester, England) 20120101 |
| Indenyl ring slippage in crown thioether complexes [IndMo(CO)2L]+ and C-S activation of trithiacyclononane: experimental and theoretical studies. | Dalton transactions (Cambridge, England : 2003) 20111028 |
| The nature of the chemical bond in linear three-body systems: from i3- to mixed chalcogen/halogen and trichalcogen moieties. | Bioinorganic chemistry and applications 20070101 |
| Transients in the oxidative and H-atom-induced degradation of 1,3,5-trithiane. Time-resolved studies in aqueous solution. | The journal of physical chemistry. A 20060727 |
| Reactions Between Chalcogen Donors and Dihalogens/Interalogens: Typology of Products and Their Characterization by FT-Raman Spectroscopy. | Bioinorganic chemistry and applications 20060101 |
| Zapoteca formosa: sulfur chemistry and phytotoxicity. | Journal of chemical ecology 20040201 |
| Design, synthesis, and in vitro biological evaluation of small molecule inhibitors of estrogen receptor alpha coactivator binding. | Journal of medicinal chemistry 20040129 |
| Lanthanum(IlI) PVC membrane electrodes based on 1,3,5-trithiacyclohexane. | Analytical chemistry 20021101 |
| The dimer, trimer and 1,2,4-trithiolane of adamantanethione. | Acta crystallographica. Section C, Crystal structure communications 20020401 |
| Calorimetric and computational study of 1,3,5-trithiane. | The Journal of organic chemistry 20010810 |
| Headspace constituents of Parkia speciosa seeds. | Natural product letters 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.