200,000+ products from a single source!
sales@angenechem.com
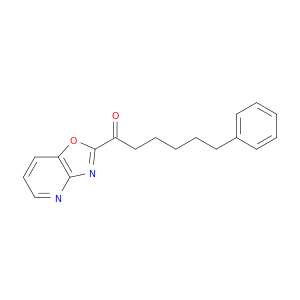
288862-83-9 | 1-Hexanone, 1-oxazolo[4,5-b]pyridin-2-yl-6-phenyl-
CAS No: 288862-83-9 Catalog No: AG002WXZ MDL No:
Product Description
Catalog Number:
AG002WXZ
Chemical Name:
1-Hexanone, 1-oxazolo[4,5-b]pyridin-2-yl-6-phenyl-
CAS Number:
288862-83-9
Molecular Formula:
C18H18N2O2
Molecular Weight:
294.3477
IUPAC Name:
1-([1,3]oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one
InChI:
InChI=1S/C18H18N2O2/c21-15(18-20-17-16(22-18)12-7-13-19-17)11-6-2-5-10-14-8-3-1-4-9-14/h1,3-4,7-9,12-13H,2,5-6,10-11H2
InChI Key:
VPZHQLPAKFVGKX-UHFFFAOYSA-N
SMILES:
O=C(c1nc2c(o1)cccn2)CCCCCc1ccccc1
Properties
Complexity:
355
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
294.137g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
294.354g/mol
Monoisotopic Mass:
294.137g/mol
Rotatable Bond Count:
7
Topological Polar Surface Area:
56A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.4
Literature
| Title | Journal |
|---|---|
| Design, synthesis and evaluation of polar head group containing 2-keto-oxazole inhibitors of FAAH. | Bioorganic & medicinal chemistry 20120115 |
| The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH). | Bioorganic & medicinal chemistry letters 20110815 |
| 1-Indol-1-yl-propan-2-ones and related heterocyclic compounds as dual inhibitors of cytosolic phospholipase A(2)alpha and fatty acid amide hydrolase. | Bioorganic & medicinal chemistry 20100115 |
| Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. | Journal of medicinal chemistry 20081211 |
| Inhibitors of proteases and amide hydrolases that employ an alpha-ketoheterocycle as a key enabling functionality. | Bioorganic & medicinal chemistry 20080215 |
| The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. | Journal of medicinal chemistry 20050811 |
| Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. | Journal of medicinal chemistry 20050324 |
| Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening: concurrent optimization of enzyme inhibitor potency and selectivity. | Bioorganic & medicinal chemistry letters 20050301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.