200,000+ products from a single source!
sales@angenechem.com
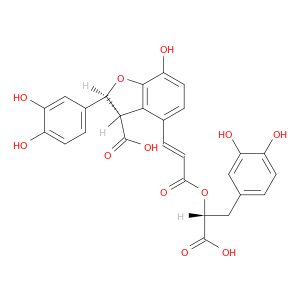
28831-65-4 | 3-Benzofurancarboxylic acid, 4-[(1E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxo-1-propen-1-yl]-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-, (2S,3S)-
CAS No: 28831-65-4 Catalog No: AG002WPO MDL No:MFCD03427311
Product Description
Catalog Number:
AG002WPO
Chemical Name:
3-Benzofurancarboxylic acid, 4-[(1E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxo-1-propen-1-yl]-2-(3,4-dihydroxyphenyl)-2,3-dihydro-7-hydroxy-, (2S,3S)-
CAS Number:
28831-65-4
Molecular Formula:
C27H22O12
Molecular Weight:
538.4564
MDL Number:
MFCD03427311
IUPAC Name:
(2S,3S)-4-[(E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxoprop-1-enyl]-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-3-carboxylic acid
InChI:
InChI=1S/C27H22O12/c28-15-5-1-12(9-18(15)31)10-20(26(34)35)38-21(33)8-4-13-2-7-17(30)25-22(13)23(27(36)37)24(39-25)14-3-6-16(29)19(32)11-14/h1-9,11,20,23-24,28-32H,10H2,(H,34,35)(H,36,37)/b8-4+/t20-,23+,24-/m1/s1
InChI Key:
UJZQBMQZMKFSRV-RGKBJLTCSA-N
SMILES:
O=C(O[C@@H](C(=O)O)Cc1ccc(c(c1)O)O)/C=C/c1ccc(c2c1[C@H](C(=O)O)[C@H](O2)c1ccc(c(c1)O)O)O
Properties
Complexity:
922
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
1
Exact Mass:
538.111g/mol
Formal Charge:
0
Heavy Atom Count:
39
Hydrogen Bond Acceptor Count:
12
Hydrogen Bond Donor Count:
7
Isotope Atom Count:
0
Molecular Weight:
538.461g/mol
Monoisotopic Mass:
538.111g/mol
Rotatable Bond Count:
9
Topological Polar Surface Area:
211A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| Incorporation of Privileged Structures into Bevirimat Can Improve Activity against Wild-Type and Bevirimat-Resistant HIV-1. | Journal of medicinal chemistry 20161013 |
| Enantioselective total synthesis of (+)-lithospermic acid. | Organic letters 20121005 |
| Synthesis of anti-HIV lithospermic acid by two diverse strategies. | Organic & biomolecular chemistry 20120728 |
| Biotransformation of salvianolic acid B by Fusarium oxysporum f. sp. Cucumerinum and its two degradation routes. | Natural product communications 20120701 |
| Improving the NQO1-inducing activities of phenolic acids from radix Salvia miltiorrhiza: a methylation strategy. | Chemical biology & drug design 20111001 |
| A concise route to dihydrobenzo[b]furans: formal total synthesis of (+)-lithospermic acid. | Organic letters 20110701 |
| Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C-H olefination. | Journal of the American Chemical Society 20110420 |
| Simultaneous determination of six phenolic constituents of Danshen injection in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. | European journal of mass spectrometry (Chichester, England) 20110101 |
| Inhibitory effects of lithospermic acid on proliferation and migration of rat vascular smooth muscle cells. | Acta pharmacologica Sinica 20090901 |
| Matrix metalloproteinase-2 inhibitors from Clinopodium chinense var. parviflorum. | Journal of natural products 20090801 |
| Microwave-assisted extraction with water for fast extraction and simultaneous RP-HPLC determination of phenolic acids in radix Salviae Miltiorrhizae. | Journal of separation science 20090701 |
| Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. | Chemico-biological interactions 20081125 |
| Three new derivatives of anti-HIV-1 polyphenols isolated from Salvia yunnanensis. | Journal of Asian natural products research 20080101 |
| Pharmacokinetics, tissue distribution, metabolism, and excretion of depside salts from Salvia miltiorrhiza in rats. | Drug metabolism and disposition: the biological fate of chemicals 20070201 |
| Hydrolytic kinetics of lithospermic acid B extracted from roots of Salvia miltiorrhiza. | Journal of pharmaceutical and biomedical analysis 20070117 |
| Clinical non-inferiority trial on treatment of coronary heart disease angina pectoris of Xin-blood stasis syndrome type with lyophilized Salvia salt of lithospermic acid powder for injection. | Chinese journal of integrative medicine 20060301 |
| Total synthesis of (+)-lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation. | Journal of the American Chemical Society 20051005 |
| The mechanisms underlying the anti-aging activity of the Chinese prescription Kangen-karyu in hydrogen peroxide-induced human fibroblasts. | The Journal of pharmacy and pharmacology 20051001 |
| Simultaneous determination of six phenolic constituents of danshen in human serum using liquid chromatography/tandem mass spectrometry. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20050605 |
| Anti-HIV activities of natural antioxidant caffeic acid derivatives: toward an antiviral supplementation diet. | Current medicinal chemistry 20050101 |
| Simultaneous determination of magnesium lithospermate B, rosmarinic acid, and lithospermic acid in beagle dog serum by liquid chromatography/tandem mass spectrometry. | Rapid communications in mass spectrometry : RCM 20040101 |
| HIV-1 integrase inhibitory substances from Coleus parvifolius. | Phytotherapy research : PTR 20030301 |
| Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. | Antiviral research 20020701 |
| Anti-lipid-peroxidative principles from Tournefortia sarmentosa. | Journal of natural products 20020501 |
| A search for anti-viral properties in Panamanian medicinal plants. The effects on HIV and its essential enzymes. | Journal of ethnopharmacology 19990101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.