200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2879-14-3
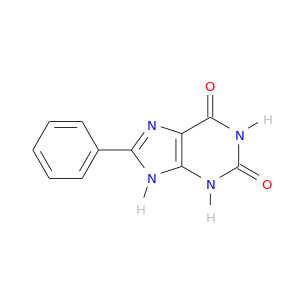
2879-14-3 | 1H-Purine-2,6-dione, 3,9-dihydro-8-phenyl-
CAS No: 2879-14-3 Catalog No: AG002WGV MDL No:
Product Description
Catalog Number:
AG002WGV
Chemical Name:
1H-Purine-2,6-dione, 3,9-dihydro-8-phenyl-
CAS Number:
2879-14-3
Molecular Formula:
C11H8N4O2
Molecular Weight:
228.2068
IUPAC Name:
8-phenyl-3,7-dihydropurine-2,6-dione
InChI:
InChI=1S/C11H8N4O2/c16-10-7-9(14-11(17)15-10)13-8(12-7)6-4-2-1-3-5-6/h1-5H,(H3,12,13,14,15,16,17)
InChI Key:
ACCCXSZCOGNLFL-UHFFFAOYSA-N
SMILES:
O=c1[nH]c2[nH]c(nc2c(=O)[nH]1)c1ccccc1
UNII:
0S2BVG6C31
Properties
Complexity:
340
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
228.065g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
228.211g/mol
Monoisotopic Mass:
228.065g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
86.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.9
Literature
| Title | Journal |
|---|---|
| Design, synthesis and inhibitory activities of 8-(substituted styrol-formamido)phenyl-xanthine derivatives on monoamine oxidase B. | Chemical & pharmaceutical bulletin 20120101 |
| Synthesis of a new series of 1H-imidazol-1-yl substituted 8-phenylxanthines as adenosine receptor ligands. | Chemistry & biodiversity 20110701 |
| Progress in the discovery of selective, high affinity A(2B) adenosine receptor antagonists as clinical candidates. | Purinergic signalling 20090301 |
| Novel selective antagonist radioligands for the pharmacological study of A(2B) adenosine receptors. | Purinergic signalling 20061101 |
| 1,8-disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. | Journal of medicinal chemistry 20020328 |
| Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. | Journal of medicinal chemistry 19931029 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







