200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 286-08-8
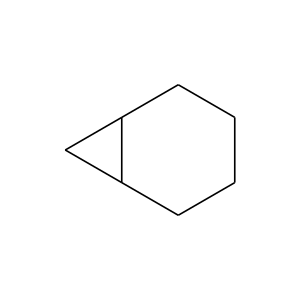
286-08-8 | Bicyclo[4.1.0]heptane
CAS No: 286-08-8 Catalog No: AG002VU2 MDL No:
Product Description
Catalog Number:
AG002VU2
Chemical Name:
Bicyclo[4.1.0]heptane
CAS Number:
286-08-8
Molecular Formula:
C7H12
Molecular Weight:
96.1702
IUPAC Name:
bicyclo[4.1.0]heptane
InChI:
InChI=1S/C7H12/c1-2-4-7-5-6(7)3-1/h6-7H,1-5H2
InChI Key:
WPHGSKGZRAQSGP-UHFFFAOYSA-N
SMILES:
C1CCC2C(C1)C2
NSC Number:
143399
Properties
Complexity:
66.1
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
96.094g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
96.173g/mol
Monoisotopic Mass:
96.094g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. | The FEBS journal 20111001 |
| Stereochemical differentiation in the Simmons-Smith reaction for cyclopropanated glucopyranose derivatives as molecular probes for glycosidases. | Bioscience, biotechnology, and biochemistry 20110101 |
| Manganese porphyrins catalyze selective C-H bond halogenations. | Journal of the American Chemical Society 20100922 |
| The effect of a complexed lithium cation on a norcarane-based radical clock. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090101 |
| Dihalocarbene insertion reactions into C-H bonds of compounds containing small rings: mechanisms and regio- and stereoselectivities. | The Journal of organic chemistry 20071026 |
| Synthesis of novel bicyclo[4.1.0]heptane and bicyclo[3.1.0]hexane derivatives as melanin-concentrating hormone receptor R1 antagonists. | Bioorganic & medicinal chemistry letters 20070901 |
| A potent bicyclic inhibitor of a family 27 alpha-galactosidase. | Organic & biomolecular chemistry 20070607 |
| Desaturase reactions complicate the use of norcarane as a mechanistic probe. Unraveling the mixture of twenty-plus products formed in enzyme-catalyzed oxidations of norcarane. | The Journal of organic chemistry 20070216 |
| Products from enzyme-catalyzed oxidations of norcarenes. | The Journal of organic chemistry 20070216 |
| Profiling mechanisms of alkane hydroxylase activity in vivo using the diagnostic substrate norcarane. | Chemistry & biology 20070201 |
| Oxygen-18 tracer studies of enzyme reactions with radical/cation diagnostic probes. | Biochemical and biophysical research communications 20051209 |
| Reaction mechanisms of non-heme diiron hydroxylases characterized in whole cells. | Journal of inorganic biochemistry 20051001 |
| Remarkable aliphatic hydroxylation by the diiron enzyme toluene 4-monooxygenase in reactions with radical or cation diagnostic probes norcarane, 1,1-dimethylcyclopropane, and 1,1-diethylcyclopropane. | Biochemistry 20041221 |
| endo/exo isomerism in norcarane and 2-norcaranol hydrotrioxides (ROOOH). | The Journal of organic chemistry 20031114 |
| Xylene monooxygenase, a membrane-spanning non-heme diiron enzyme that hydroxylates hydrocarbons via a substrate radical intermediate. | Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry 20030901 |
| Evaluation of norcarane as a probe for radicals in cytochome p450- and soluble methane monooxygenase-catalyzed hydroxylation reactions. | Journal of the American Chemical Society 20020619 |
| Revisiting the mechanism of P450 enzymes with the radical clocks norcarane and spiro[2,5]octane. | Journal of the American Chemical Society 20020529 |
| Intermediate Q from soluble methane monooxygenase hydroxylates the mechanistic substrate probe norcarane: evidence for a stepwise reaction. | Journal of the American Chemical Society 20011205 |
Related Products
© 2019 Angene International Limited. All rights Reserved.