200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 285-76-7
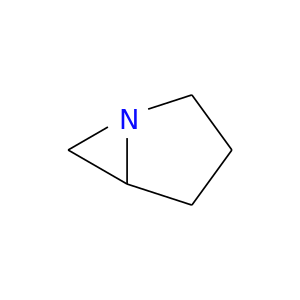
285-76-7 | 1-Azabicyclo[3.1.0]hexane
CAS No: 285-76-7 Catalog No: AG002VJH MDL No:
Product Description
Catalog Number:
AG002VJH
Chemical Name:
1-Azabicyclo[3.1.0]hexane
CAS Number:
285-76-7
Molecular Formula:
C5H9N
Molecular Weight:
83.1317
IUPAC Name:
1-azabicyclo[3.1.0]hexane
InChI:
InChI=1S/C5H9N/c1-2-5-4-6(5)3-1/h5H,1-4H2
InChI Key:
QRDSDKAGXMWBID-UHFFFAOYSA-N
SMILES:
C1CN2C(C1)C2
Properties
Complexity:
70.3
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
83.073g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
83.134g/mol
Monoisotopic Mass:
83.073g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
3A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
0.6
Literature
| Title | Journal |
|---|---|
| Mode of action and biosynthesis of the azabicycle-containing natural products azinomycin and ficellomycin. | Natural product reports 20110401 |
| Synthesis and structure-activity relationship of N-(3-azabicyclo[3.1.0]hex-6-ylmethyl)-5-(2-pyridinyl)-1,3-thiazol-2-amines derivatives as NPY Y5 antagonists. | Bioorganic & medicinal chemistry letters 20100815 |
| Synthesis and reaction of 1-azabicyclo[3.1.0]hexane. | Chemical & pharmaceutical bulletin 20091001 |
| Conformational constraint in oxazolidinone antibacterials. Synthesis and structure-activity studies of (azabicyclo[3.1.0]hexylphenyl)oxazolidinones. | Journal of medicinal chemistry 20050728 |
| 'Broad spectrum' antidepressants: is more better for the treatment of depression? | Life sciences 20031107 |
| Mass spectral fragmentation of (S,S)-2-substituted 4,4-diphenyl-3,1-oxazabicyclo[3.3.0]octanes: ring contraction of pyrrolidine and 1,3-oxazolidine in mass spectrometry. | Rapid communications in mass spectrometry : RCM 20030101 |
| A method for the parallel synthesis of multiply substituted oxazolidinones. | Journal of combinatorial chemistry 20020101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.