200,000+ products from a single source!
sales@angenechem.com
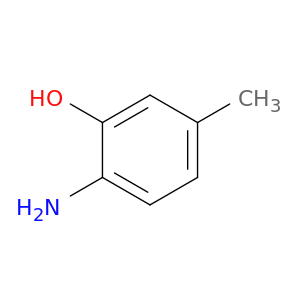
2835-98-5 | 2-Amino-5-methylphenol
CAS No: 2835-98-5 Catalog No: AG003GHE MDL No:MFCD00007693
Product Description
Catalog Number:
AG003GHE
Chemical Name:
2-Amino-5-methylphenol
CAS Number:
2835-98-5
Molecular Formula:
C7H9NO
Molecular Weight:
123.1525
MDL Number:
MFCD00007693
IUPAC Name:
2-amino-5-methylphenol
InChI:
InChI=1S/C7H9NO/c1-5-2-3-6(8)7(9)4-5/h2-4,9H,8H2,1H3
InChI Key:
HCPJEHJGFKWRFM-UHFFFAOYSA-N
SMILES:
Cc1ccc(c(c1)O)N
EC Number:
220-620-7
UNII:
QCG4ES2A26
NSC Number:
322874
Properties
Complexity:
94.9
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
123.068g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
123.155g/mol
Monoisotopic Mass:
123.068g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
46.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Peculiarity of methoxy group-substituted phenylhydrazones in Fischer indole synthesis. | Proceedings of the Japan Academy. Series B, Physical and biological sciences 20120111 |
| 2-[(Naphthalen-1-yl-methyl-idene)amino]-5-methyl-phenol. | Acta crystallographica. Section E, Structure reports online 20110901 |
| 2-Aminophenoxazine-3-one and 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one cause cellular apoptosis by reducing higher intracellular pH in cancer cells. | Proceedings of the Japan Academy. Series B, Physical and biological sciences 20110511 |
| Vibrational spectroscopy investigation using ab initio and density functional theory analysis on the structure of 2-amino-5-methylphenol. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20070601 |
| Effect of natural phenol derivatives on skeletal type sarcoplasmic reticulum Ca2+ -ATPase and ryanodine receptor. | Journal of muscle research and cell motility 20070101 |
| A novel kinetic determination of dissolved chromium species in natural and industrial waste water. | Talanta 20060915 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Final report on the safety assessment of 6-Amino-m-Cresol, 6-Amino-o-Cresol, 4-Amino-m-Cresol, 5-Amino-4-Chloro-o-Cresol, 5-Amino-6-Chloro-o-Cresol, and 4-Chloro-2-Aminophenol. | International journal of toxicology 20040101 |
| Antiviral activity of 2-amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one on poliovirus. | The Tohoku journal of experimental medicine 20030701 |
| A novel phenoxazine derivative suppresses proliferation of human endometrial adenocarcinoma cell lines, inducing G2M accumulation and apoptosis. | Oncology reports 20030101 |
| A novel meta-cleavage dioxygenase that cleaves a carboxyl-group-substituted 2-aminophenol. Purification and characterization of 4-amino-3-hydroxybenzoate 2,3-dioxygenase from Bordetella sp. strain 10d. | European journal of biochemistry 20021201 |
| Antitumor activity of a phenoxazine compound, 2-amino-4,4alpha-dihydro-4alpha,7-dimethyl-3H-phenoxazine-3-one against human B cell and T cell lymphoblastoid cell lines: induction of mixed types of cell death, apoptosis, and necrosis. | Journal of cancer research and clinical oncology 20020701 |
| An improved method for the rapid preparation of 2-amino-4,4a-dihydro-4a,7-dimethyl-3H-phenoxazine-3-one, a novel antitumor agent. | Bioorganic & medicinal chemistry letters 20010423 |
| Prevention of growth of human lung carcinoma cells and induction of apoptosis by a novel phenoxazinone, 2-amino-4,4alpha-dihydro-4alpha,7-dimethyl-3H-phenoxazine-3-one. | Anti-cancer drugs 20010401 |
| Antitumor effects of a novel phenoxazine derivative on human leukemia cell lines in vitro and in vivo. | Clinical cancer research : an official journal of the American Association for Cancer Research 20010301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.