200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 279-40-3
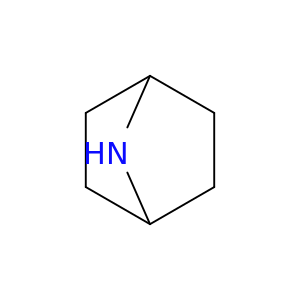
279-40-3 | 7-Azabicyclo[2.2.1]heptane
CAS No: 279-40-3 Catalog No: AG003ND3 MDL No:MFCD13624216
Product Description
Catalog Number:
AG003ND3
Chemical Name:
7-Azabicyclo[2.2.1]heptane
CAS Number:
279-40-3
Molecular Formula:
C6H11N
Molecular Weight:
97.1582
MDL Number:
MFCD13624216
IUPAC Name:
7-azabicyclo[2.2.1]heptane
InChI:
InChI=1S/C6H11N/c1-2-6-4-3-5(1)7-6/h5-7H,1-4H2
InChI Key:
SNZSSCZJMVIOCR-UHFFFAOYSA-N
SMILES:
C1CC2NC1CC2
Properties
Complexity:
62.2
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
97.089g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
97.161g/mol
Monoisotopic Mass:
97.089g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
12A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.9
Literature
| Title | Journal |
|---|---|
| 7-Azabicyclo[2.2.1]heptane as a scaffold for the development of selective sigma-2 (σ2) receptor ligands. | Bioorganic & medicinal chemistry letters 20120615 |
| Synthesis and binding affinity at α4β2 and α7 nicotinic acetylcholine receptors of new analogs of epibatidine and epiboxidine containing the 7-azabicyclo[2.2.1]hept-2-ene ring system. | Bioorganic & medicinal chemistry letters 20120115 |
| 7-azabicyclo[2.2.1]heptane as a structural motif to block mutagenicity of nitrosamines. | Bioorganic & medicinal chemistry 20110415 |
| Asymmetric synthesis of 3,4-diaminocyclohexanol and endo-7-azabicyclo[2.2.1]heptan-2-amine. | Organic letters 20101105 |
| Water-stable helical structure of tertiary amides of bicyclic β-amino acid bearing 7-azabicyclo[2.2.1]heptane. Full control of amide cis-trans equilibrium by bridgehead substitution. | Journal of the American Chemical Society 20101027 |
| Mechanistic analysis of intramolecular free radical reactions toward synthesis of 7-azabicyclo[2.2.1]heptane derivatives. | The Journal of organic chemistry 20090605 |
| A novel intramolecular Ugi reaction with 7-azabicyclo[2.2.1]heptane derivatives followed by post-condensation acylations: a new entry to azanorbornyl peptidomimetics. | Organic & biomolecular chemistry 20090121 |
| 7-Azabicyclo[2.2.1]heptane as a unique and effective dialkylamino auxochrome moiety: demonstration in a fluorescent rhodamine dye. | Journal of the American Chemical Society 20081231 |
| Nonplanar structures of thioamides derived from 7-azabicyclo[2.2.1]heptane. Electronically tunable planarity of thioamides. | The Journal of organic chemistry 20081121 |
| Synthesis of heterocyclic analogues of epibatidine via 7-azabicyclo[2.2.1]hept-2-yl radical intermediates. 1. Intermolecular reactions. | The Journal of organic chemistry 20080905 |
| Structure-activity relationships of adenosines with heterocyclic N6-substituents. | Bioorganic & medicinal chemistry letters 20071215 |
| Synthesis and nicotinic acetylcholine receptor binding properties of bridged and fused ring analogues of epibatidine. | Journal of medicinal chemistry 20071213 |
| Synthesis of 7-azabicyclo[2.2.1]heptane and 2-oxa-4-azabicyclo[3.3.1]non-3-ene derivatives by base-promoted heterocyclization of alkyl N-(cis(trans)-3,trans(cis)-4-dibromocyclohex-1-yl)carbamates and N-(cis(trans)-3,trans(cis)-4-dibromocyclohex-1-yl)-2,2,2-trifluoroacetamides. | The Journal of organic chemistry 20071109 |
| Aza-Prins-pinacol approach to 7-azabicyclo[2.2.1]heptanes: syntheses of (+/-)-epibatidine and (+/-)-epiboxidine. | The Journal of organic chemistry 20071012 |
| Unusually high pyramidal geometry of the bicyclic amide nitrogen in a complex 7-azabicyclo[2.2.1]heptane derivative: Theoretical analysis using a bottom-up strategy. | The journal of physical chemistry. B 20050616 |
| aza-Prins-pinacol approach to 7-azabicyclo[2.2.1]heptanes and ring expansion to [3.2.1]tropanes. | Organic letters 20050331 |
| Cycloadditions of 2-azaallyllithium species with conjugated polyenes. | The Journal of organic chemistry 20040220 |
| An evaluation of amide group planarity in 7-azabicyclo[2.2.1]heptane amides. Low amide bond rotation barrier in solution. | Journal of the American Chemical Society 20031210 |
| Synthesis of enantiomerically pure 1,2-diamine derivatives of 7-azabicyclo[2.2.1]heptane. New leads as glycosidase inhibitors and rigid scaffolds for the preparation of peptide analogues. | The Journal of organic chemistry 20030711 |
| Synthesis and biological evaluation at nicotinic acetylcholine receptors of N-arylalkyl- and N-aryl-7-azabicyclo[2.2.1]heptanes. | Journal of medicinal chemistry 20020704 |
| Structural features of aliphatic N-nitrosamines of 7-azabicyclo[2.2.1]heptanes that facilitate N-NO bond cleavage. | Journal of the American Chemical Society 20011024 |
| Synthesis of n-heteroaryl-7-azabicyclo[2.2.1]heptane derivatives via palladium-bisimidazol-2-ylidene complex catalyzed amination reactions. | Organic letters 20010503 |
| exo-2-(Pyridazin-4-yl)-7-azabicyclo[2.2.1]heptanes: syntheses and nicotinic acetylcholine receptor agonist activity of potent pyridazine analogues of (+/-)-epibatidine. | Journal of medicinal chemistry 20010104 |
| [Nitrogen pyramidal amides and related compounds]. | Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 20010101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.