200,000+ products from a single source!
sales@angenechem.com
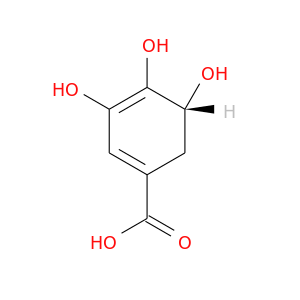
27655-56-7 | 1,3-Cyclohexadiene-1-carboxylic acid, 3,4,5-trihydroxy-, (R)- (9CI)
CAS No: 27655-56-7 Catalog No: AG002UC9 MDL No:
Product Description
Catalog Number:
AG002UC9
Chemical Name:
1,3-Cyclohexadiene-1-carboxylic acid, 3,4,5-trihydroxy-, (R)- (9CI)
CAS Number:
27655-56-7
Molecular Formula:
C7H8O5
Molecular Weight:
172.1354
IUPAC Name:
(5R)-3,4,5-trihydroxycyclohexa-1,3-diene-1-carboxylic acid
InChI:
InChI=1S/C7H8O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1,5,8-10H,2H2,(H,11,12)/t5-/m1/s1
InChI Key:
HFMGXSXAGHUXTI-RXMQYKEDSA-N
SMILES:
OC(=O)C1=CC(=C([C@@H](C1)O)O)O
Properties
Complexity:
276
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
172.037g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
172.136g/mol
Monoisotopic Mass:
172.037g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
98A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1
Literature
| Title | Journal |
|---|---|
| Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). | Plant molecular biology 20110401 |
| Insights into the mechanism of type I dehydroquinate dehydratases from structures of reaction intermediates. | The Journal of biological chemistry 20110204 |
| Conversion of quinate to 3-dehydroshikimate by Ca-alginate-immobilized membrane of Gluconobacter oxydans IFO 3244 and subsequent asymmetric reduction of 3-dehydroshikimate to shikimate by immobilized cytoplasmic NADP-shikimate dehydrogenase. | Bioscience, biotechnology, and biochemistry 20100101 |
| Structural studies of shikimate dehydrogenase from Bacillus anthracis complexed with cofactor NADP. | Journal of molecular modeling 20090201 |
| The missing link in petrobactin biosynthesis: asbF encodes a (-)-3-dehydroshikimate dehydratase. | Biochemistry 20081125 |
| A novel 3-dehydroquinate dehydratase catalyzing extracellular formation of 3-dehydroshikimate by oxidative fermentation of Gluconobacter oxydans IFO 3244. | Bioscience, biotechnology, and biochemistry 20080601 |
| Directed evolution of 2-keto-3-deoxy-6-phosphogalactonate aldolase to replace 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthase. | Journal of the American Chemical Society 20070516 |
| Enzymatic preparation of metabolic intermediates, 3-dehydroquinate and 3-dehydroshikimate, in the shikimate pathway. | Bioscience, biotechnology, and biochemistry 20061201 |
| Benzene-free synthesis of catechol: interfacing microbial and chemical catalysis. | Journal of the American Chemical Society 20050309 |
| A thermostable shikimate 5-dehydrogenase from the archaeon Archaeoglobus fulgidus. | FEMS microbiology letters 20040901 |
| Conversion of (-)-3-dehydroshikimic acid into derivatives of the (+)-enantiomer. | The Journal of organic chemistry 20030822 |
| The 2.3-A crystal structure of the shikimate 5-dehydrogenase orthologue YdiB from Escherichia coli suggests a novel catalytic environment for an NAD-dependent dehydrogenase. | The Journal of biological chemistry 20030523 |
| Antioxidant activity of 3-dehydroshikimic acid in liposomes, emulsions, and bulk oil. | Journal of agricultural and food chemistry 20030423 |
| Altered glucose transport and shikimate pathway product yields in E. coli. | Biotechnology progress 20030101 |
| Pulse experiments as a prerequisite for the quantification of in vivo enzyme kinetics in aromatic amino acid pathway of Escherichia coli. | Biotechnology progress 20020101 |
| Modulation of phosphoenolpyruvate synthase expression increases shikimate pathway product yields in E. coli. | Biotechnology progress 20020101 |
| Hydroaromatic equilibration during biosynthesis of shikimic acid. | Journal of the American Chemical Society 20011024 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







