200,000+ products from a single source!
sales@angenechem.com
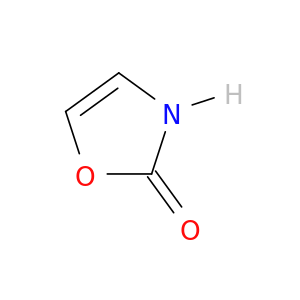
27584-70-9 | 2(3H)-Oxazolone
CAS No: 27584-70-9 Catalog No: AG002U5X MDL No:MFCD00075369
Product Description
Catalog Number:
AG002U5X
Chemical Name:
2(3H)-Oxazolone
CAS Number:
27584-70-9
Molecular Formula:
C3H3NO2
Molecular Weight:
85.0614
MDL Number:
MFCD00075369
IUPAC Name:
3H-1,3-oxazol-2-one
InChI:
InChI=1S/C3H3NO2/c5-3-4-1-2-6-3/h1-2H,(H,4,5)
InChI Key:
XYVMOLOUBJBNBF-UHFFFAOYSA-N
SMILES:
O=c1occ[nH]1
Properties
Complexity:
97
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
85.016g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
85.062g/mol
Monoisotopic Mass:
85.016g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
38.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.3
Literature
| Title | Journal |
|---|---|
| A novel ring oxidation of 4- or 5-substituted 2H-oxazole to corresponding 2-oxazolone catalyzed by cytosolic aldehyde oxidase. | Drug metabolism and disposition: the biological fate of chemicals 20120901 |
| Design, synthesis and evaluation of polar head group containing 2-keto-oxazole inhibitors of FAAH. | Bioorganic & medicinal chemistry 20120115 |
| Highly regio- and stereoselective Diels-Alder cycloadditions via two-step and multicomponent reactions promoted by infrared irradiation under solvent-free conditions. | International journal of molecular sciences 20120101 |
| Rhodium-catalyzed decarboxylative cycloaddition route to substituted anilines. | The Journal of organic chemistry 20110603 |
| Exacerbation of oxazolone colitis by infection with the helminth Hymenolepis diminuta: involvement of IL-5 and eosinophils. | The American journal of pathology 20101201 |
| Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators. | The Journal of allergy and clinical immunology 20100101 |
| Novel ketooxazole based inhibitors of fatty acid amide hydrolase (FAAH). | Bioorganic & medicinal chemistry letters 20080315 |
| An unprecedented Pd-catalyzed, water-promoted sequential oxidative aminocarbonylation-cyclocarbonylation process leading to 2-oxazolidinones. | Organic letters 20070816 |
| Use of the oxazole-olefin diels-alder reaction in the total synthesis of the monoterpene alkaloids (-)-plectrodorine and (+)-oxerine. | Chemical & pharmaceutical bulletin 20060101 |
| Elucidation of fatty acid amide hydrolase inhibition by potent alpha-ketoheterocycle derivatives from Monte Carlo simulations. | Journal of the American Chemical Society 20051214 |
| Studies on the total synthesis of streptazolin and its related natural products: first total synthesis of (+/-)-8alpha-hydroxystreptazolone. | The Journal of organic chemistry 20040319 |
| Histone deacetylase inhibitors: the Abbott experience. | Current medicinal chemistry 20031101 |
| Total synthesis of (+/-)-8alpha-hydroxystreptazolone. | Organic letters 20021128 |
| Asymmetric Diels-Alder, Michael, and aldol reactions using a planar chiral 1,3-oxazol-2(3H)-one derived from (R)-(+)-4-hydroxy-[2.2]paracyclophane. | The Journal of organic chemistry 20020419 |
| Highly accelerating effect of lewis acids on ruthenium(II)-catalyzed radical addition reactions. | Chemical & pharmaceutical bulletin 20020301 |
| Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. | Journal of immunology (Baltimore, Md. : 1950) 20020215 |
| Versatile synthons for optically pure alpha-amino aldehydes and alpha-amino acids: (+)- and (-)-4,5-dialkoxy-2-oxazolidinones. | Organic letters 20010322 |
| Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. | Journal of immunology (Baltimore, Md. : 1950) 20010315 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







