200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 27516-53-6

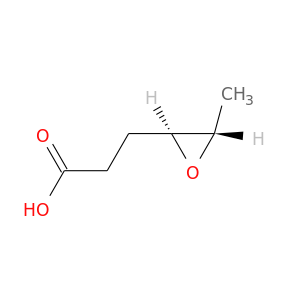
27516-53-6 | Pentenoic acid
CAS No: 27516-53-6 Catalog No: AG002TZG MDL No:
Product Description
Catalog Number:
AG002TZG
Chemical Name:
Pentenoic acid
CAS Number:
27516-53-6
Molecular Formula:
C5H8O2
Molecular Weight:
100.1158
IUPAC Name:
pent-2-enoic acid
InChI:
InChI=1S/C5H8O2/c1-2-3-4-5(6)7/h3-4H,2H2,1H3,(H,6,7)
InChI Key:
YIYBQIKDCADOSF-UHFFFAOYSA-N
SMILES:
CCC=CC(=O)O
Properties
Complexity:
84.1
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
100.052g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
100.117g/mol
Monoisotopic Mass:
100.052g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
37.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
1
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| Structure-activity relationship (SAR) for the prediction of gas-phase ozonolysis rate coefficients: an extension towards heteroatomic unsaturated species. | Physical chemistry chemical physics : PCCP 20110221 |
| Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. | PloS one 20110101 |
| Enhancement of the click chemistry for the inverse Diels Alder technology by functionalization of amide-based monomers. | International journal of medical sciences 20110101 |
| γ-Valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. | Langmuir : the ACS journal of surfaces and colloids 20101102 |
| A new labdanic norditerpene from Pinus sylvestris. | Natural product research 20101001 |
| Pentenoic acid pathways for cellulosic biofuels. | Angewandte Chemie (International ed. in English) 20100614 |
| Extension of the PNA world by functionalized PNA monomers eligible candidates for inverse Diels Alder Click Chemistry. | International journal of medical sciences 20100101 |
| Towards 'bio-based' Nylon: conversion of gamma-valerolactone to methyl pentenoate under catalytic distillation conditions. | Chemical communications (Cambridge, England) 20070907 |
| Syntrophomonas palmitatica sp. nov., an anaerobic, syntrophic, long-chain fatty-acid-oxidizing bacterium isolated from methanogenic sludge. | International journal of systematic and evolutionary microbiology 20070901 |
| Efficient synthesis of a trisubstituted 1,6-naphthyridone from acetonedicarboxylate and regioselective Suzuki arylation. | The Journal of organic chemistry 20051209 |
| Conjugate additions of Me2CuLi to enantiopure gamma-hydroxy-delta-sulfinyl and sulfonyl pentenoates. | The Journal of organic chemistry 20051125 |
| Syntrophomonas erecta sp. nov., a novel anaerobe that syntrophically degrades short-chain fatty acids. | International journal of systematic and evolutionary microbiology 20050301 |
| Asymmetric total synthesis of bacillariolide III, a marine oxylipin. | Organic letters 20040205 |
| Intramolecularly competitive Ireland-Claisen rearrangements: scope and potential applications to natural product synthesis. | The Journal of organic chemistry 20020405 |
Related Products
© 2019 Angene International Limited. All rights Reserved.