200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 274-95-3
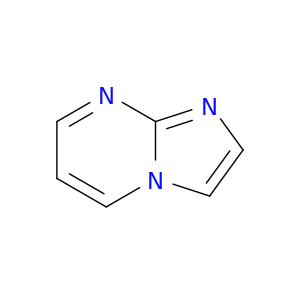
274-95-3 | Imidazo[1,2-a]pyrimidine
CAS No: 274-95-3 Catalog No: AG003QZL MDL No:MFCD06245377
Product Description
Catalog Number:
AG003QZL
Chemical Name:
Imidazo[1,2-a]pyrimidine
CAS Number:
274-95-3
Molecular Formula:
C6H5N3
Molecular Weight:
119.1240
MDL Number:
MFCD06245377
IUPAC Name:
imidazo[1,2-a]pyrimidine
InChI:
InChI=1S/C6H5N3/c1-2-7-6-8-3-5-9(6)4-1/h1-5H
InChI Key:
INSWZAQOISIYDT-UHFFFAOYSA-N
SMILES:
c1ccn2c(n1)ncc2
Properties
Complexity:
105
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
119.048g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
119.127g/mol
Monoisotopic Mass:
119.048g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
30.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. | Bioorganic & medicinal chemistry 20120401 |
| Lifelong CRF overproduction is associated with altered gene expression and sensitivity of discrete GABA(A) and mGlu receptor subtypes. | Psychopharmacology 20120201 |
| Systematic structure modifications of imidazo[1,2-a]pyrimidine to reduce metabolism mediated by aldehyde oxidase (AO). | Journal of medicinal chemistry 20111110 |
| Structure-activity relationship of 4(5)-aryl-2-amino-1H-imidazoles, N1-substituted 2-aminoimidazoles and imidazo[1,2-a]pyrimidinium salts as inhibitors of biofilm formation by Salmonella typhimurium and Pseudomonas aeruginosa. | Journal of medicinal chemistry 20110127 |
| Post Groebke-Blackburn multicomponent protocol: synthesis of new polyfunctional imidazo[1,2-a]pyridine and imidazo[1,2-a]pyrimidine derivatives as potential antimicrobial agents. | European journal of medicinal chemistry 20101201 |
| 5-HT1A receptor blockade reverses GABA(A) receptor alpha3 subunit-mediated anxiolytic effects on stress-induced hyperthermia. | Psychopharmacology 20100801 |
| Efficient one-pot, two-step, microwave-assisted procedure for the synthesis of polysubstituted 2-aminoimidazoles. | Organic letters 20061207 |
| 8-Fluoroimidazo[1,2-a]pyridine: synthesis, physicochemical properties and evaluation as a bioisosteric replacement for imidazo[1,2-a]pyrimidine in an allosteric modulator ligand of the GABA A receptor. | Bioorganic & medicinal chemistry letters 20060315 |
| Palladium-catalyzed regioselective arylation of imidazo[1,2-a]pyrimidine. | Organic letters 20031211 |
| Effect of imidazo[1,2-a]pyrimidine derivatives on leukocyte function. | Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20010601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.