200,000+ products from a single source!
sales@angenechem.com
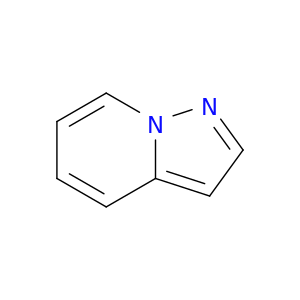
274-56-6 | Pyrazolo[1,5-a]pyridine
CAS No: 274-56-6 Catalog No: AG003841 MDL No:MFCD08752622
Product Description
Catalog Number:
AG003841
Chemical Name:
Pyrazolo[1,5-a]pyridine
CAS Number:
274-56-6
Molecular Formula:
C7H6N2
Molecular Weight:
118.1359
MDL Number:
MFCD08752622
IUPAC Name:
pyrazolo[1,5-a]pyridine
InChI:
InChI=1S/C7H6N2/c1-2-6-9-7(3-1)4-5-8-9/h1-6H
InChI Key:
DVUBDHRTVYLIPA-UHFFFAOYSA-N
SMILES:
c1ccc2n(c1)ncc2
Properties
Complexity:
103
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
118.053g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
118.139g/mol
Monoisotopic Mass:
118.053g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Phosphodiesterase inhibitors. Part 4: design, synthesis and structure-activity relationships of dual PDE3/4-inhibitory fused bicyclic heteroaromatic-4,4-dimethylpyrazolones. | Bioorganic & medicinal chemistry letters 20120915 |
| Au(I)-catalyzed and iodine-mediated cyclization of enynylpyrazoles to provide pyrazolo[1,5-a]pyridines. | Organic & biomolecular chemistry 20120907 |
| Synthesis and structure-activity relationships of pyrazolo[1,5-a]pyridine derivatives: potent and orally active antagonists of corticotropin-releasing factor 1 receptor. | Journal of medicinal chemistry 20120614 |
| Phosphodiesterase inhibitors. Part 3: Design, synthesis and structure-activity relationships of dual PDE3/4-inhibitory fused bicyclic heteroaromatic-dihydropyridazinones with anti-inflammatory and bronchodilatory activity. | Bioorganic & medicinal chemistry 20120301 |
| Ultrasound assisted one-pot, three-components synthesis of pyrimido[1,2-a]benzimidazoles and pyrazolo[3,4-b]pyridines: A new access via phenylsulfone synthon. | Ultrasonics sonochemistry 20120101 |
| Discovery of pyrazolo[1,5-a]pyridines as p110α-selective PI3 kinase inhibitors. | Bioorganic & medicinal chemistry 20120101 |
| Novel pyrazolo[1,5-a]pyridines as p110α-selective PI3 kinase inhibitors: Exploring the benzenesulfonohydrazide SAR. | Bioorganic & medicinal chemistry 20120101 |
| Synthesis, X-ray crystal structure and fluorescent spectra of novel pyrazolo[1,5-a]pyrazin-4(5H)-one derivatives. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20111015 |
| Phosphodiesterase inhibitors. Part 2: design, synthesis, and structure-activity relationships of dual PDE3/4-inhibitory pyrazolo[1,5-a]pyridines with anti-inflammatory and bronchodilatory activity. | Bioorganic & medicinal chemistry letters 20110915 |
| A copper-mediated cyclization reaction of hydrazine with enediynones providing pyrazolo[1,5-a]pyridines. | Organic & biomolecular chemistry 20110207 |
| One-step synthesis of diarylpyrazolo[3,4-b]pyridines from isoflavones. | Journal of combinatorial chemistry 20100712 |
| Ethyl 3-{5-[(diethyl-amino)meth-yl]isoxazol-3-yl}-2-phenyl-pyrazolo[1,5-a]pyridine-5-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20100401 |
| Assessment of the putative binding conformation of a pyrazolopyridine class of inhibitors of MAPKAPK2 using computational studies. | European journal of medicinal chemistry 20100101 |
| The first examples of chemical modification of oligomycin A. | The Journal of antibiotics 20100101 |
| Novel pyrazolopyrimidines as highly potent B-Raf inhibitors. | Bioorganic & medicinal chemistry letters 20091215 |
| An efficient one-step synthesis of heterobiaryl pyrazolo[3,4-b]pyridines via indole ring opening. | Organic letters 20091119 |
| Structure-activity relationship study of EphB3 receptor tyrosine kinase inhibitors. | Bioorganic & medicinal chemistry letters 20091101 |
| Exploiting the pyrazolo[3,4-d]pyrimidin-4-one ring system as a useful template to obtain potent adenosine deaminase inhibitors. | Journal of medicinal chemistry 20090326 |
| The identification of pyrazolo[1,5-a]pyridines as potent p38 kinase inhibitors. | Bioorganic & medicinal chemistry letters 20081015 |
| Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. | Bioorganic & medicinal chemistry letters 20080801 |
| Substituted pyrazolo[3,4-b]pyridines as potent A1 adenosine antagonists: synthesis, biological evaluation, and development of an A1 bovine receptor model. | ChemMedChem 20080601 |
| Design, synthesis, and biological evaluation of AT1 angiotensin II receptor antagonists based on the pyrazolo[3,4-b]pyridine and related heteroaromatic bicyclic systems. | Journal of medicinal chemistry 20080410 |
| Synthesis, radiofluorination, and in vitro evaluation of pyrazolo[1,5-a]pyridine-based dopamine D4 receptor ligands: discovery of an inverse agonist radioligand for PET. | Journal of medicinal chemistry 20080327 |
| Concise routes to pyrazolo[1,5-a]pyridin-3-yl pyridazin-3-ones. | Organic & biomolecular chemistry 20080107 |
| Pyrazolo[1,5-a]pyrimidines as orally available inhibitors of cyclin-dependent kinase 2. | Bioorganic & medicinal chemistry letters 20071115 |
| Rapid hit to lead evaluation of pyrazolo[3,4-d]pyrimidin-4-one as selective and orally bioavailable mGluR1 antagonists. | Bioorganic & medicinal chemistry letters 20070801 |
| Synthesis and evaluation of pyrazolo[3,4-b]pyridine CDK1 inhibitors as anti-tumor agents. | Bioorganic & medicinal chemistry letters 20070801 |
| Pyrazolo[1,5-a]pyridine antiherpetics: effects of the C3 substituent on antiviral activity. | Bioorganic & medicinal chemistry letters 20070515 |
| Synthesis of C-6 substituted pyrazolo[1,5-a]pyridines with potent activity against herpesviruses. | Bioorganic & medicinal chemistry 20060215 |
| Pyrazoloheteroaryls: novel p38alpha MAP kinase inhibiting scaffolds with oral activity. | Bioorganic & medicinal chemistry letters 20060115 |
| Tissue distribution of radioiodinated FAUC113: assessment of a pyrazolo(1,5-a) pyridine based dopamine D4 receptor radioligand candidate. | Nuklearmedizin. Nuclear medicine 20060101 |
| Synthesis of novel substituted 2-phenylpyrazolopyridines with potent activity against herpesviruses. | Bioorganic & medicinal chemistry 20050915 |
| New pyrazolo[3,4-b]pyridones as selective A(1) adenosine receptor antagonists: synthesis, biological evaluation and molecular modelling studies. | Organic & biomolecular chemistry 20050621 |
| Pyrazolopyridine antiherpetics: SAR of C2' and C7 amine substituents. | Bioorganic & medicinal chemistry 20050401 |
| Synthesis and biological evaluation of some acyclic 4,6-disubstituted 1H-pyrazolo[3,4-d]pyrimidine nucleosides. | Nucleosides, nucleotides & nucleic acids 20030101 |
| Di- and trisubstituted pyrazolo[1,5-a]pyridine derivatives: synthesis, dopamine receptor binding and ligand efficacy. | Bioorganic & medicinal chemistry letters 20020225 |
Related Products
© 2019 Angene International Limited. All rights Reserved.