200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 274-47-5
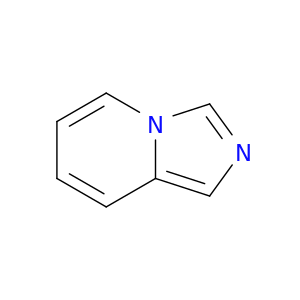
274-47-5 | Imidazolo[1,5-a]pyridine
CAS No: 274-47-5 Catalog No: AG007KV3 MDL No:MFCD03407375
Product Description
Catalog Number:
AG007KV3
Chemical Name:
Imidazolo[1,5-a]pyridine
CAS Number:
274-47-5
Molecular Formula:
C7H6N2
Molecular Weight:
118.1359
MDL Number:
MFCD03407375
IUPAC Name:
imidazo[1,5-a]pyridine
InChI:
InChI=1S/C7H6N2/c1-2-4-9-6-8-5-7(9)3-1/h1-6H
InChI Key:
JMANUKZDKDKBJP-UHFFFAOYSA-N
SMILES:
c1ccc2n(c1)cnc2
NSC Number:
119858
Properties
Complexity:
103
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
118.053g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
118.139g/mol
Monoisotopic Mass:
118.053g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.8
Literature
| Title | Journal |
|---|---|
| Synthesis, characterization, optical properties and theoretical studies of novel substituted imidazo[1,5-a]pyridinyl 1,3,4-oxadiazole derivatives. | Journal of fluorescence 20121101 |
| Fluorescence switching of imidazo[1,5-a]pyridinium ions: pH-sensors with dual emission pathways. | Organic letters 20120615 |
| Studies toward the total synthesis of nagelamide K. | Organic letters 20120420 |
| Efficient, single-step access to imidazo[1,5-a]pyridine n-heterocyclic carbene precursors. | Organic letters 20111007 |
| Multicomponent reaction of imidazo[1,5-a]pyridine carbenes with aldehydes and dimethyl acetylenedicarboxylate or allenoates: a straightforward approach to fully substituted furans. | The Journal of organic chemistry 20101001 |
| Orthogonal synthesis of densely functionalized pyrroles and thiophenes from the reactions of imidazo[1,5-a]pyridine carbene-derived zwitterions with electron-deficient alkynes. | The Journal of organic chemistry 20100402 |
| Pyrido[1,2-a][1,2,4]triazol-3-ylidenes as a new family of stable annulated N-heterocyclic carbenes: synthesis, reactivity, and their application in coordination chemistry and organocatalysis. | The Journal of organic chemistry 20081107 |
| Synthesis of 2-azaindolizines by using an iodine-mediated oxidative desulfurization promoted cyclization of N-2-pyridylmethyl thioamides and an investigation of their photophysical properties. | Organic letters 20061123 |
| Imidazo[1,5-a]pyridine: a versatile architecture for stable N-heterocyclic carbenes. | Journal of the American Chemical Society 20050316 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.