200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 272-49-1
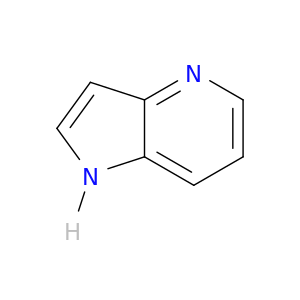
272-49-1 | 4-Azaindole
CAS No: 272-49-1 Catalog No: AG0033WX MDL No:MFCD00971977
Product Description
Catalog Number:
AG0033WX
Chemical Name:
4-Azaindole
CAS Number:
272-49-1
Molecular Formula:
C7H6N2
Molecular Weight:
118.1359
MDL Number:
MFCD00971977
IUPAC Name:
1H-pyrrolo[3,2-b]pyridine
InChI:
InChI=1S/C7H6N2/c1-2-6-7(8-4-1)3-5-9-6/h1-5,9H
InChI Key:
XWIYUCRMWCHYJR-UHFFFAOYSA-N
SMILES:
c1cnc2c(c1)[nH]cc2
Properties
Complexity:
103
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
118.053g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
118.139g/mol
Monoisotopic Mass:
118.053g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
28.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Inhibitors of HIV-1 attachment. Part 10. The discovery and structure-activity relationships of 4-azaindole cores. | Bioorganic & medicinal chemistry letters 20130101 |
| Spectroscopic study of the ground and excited state prototropic equilibria of 4-azaindole. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20121101 |
| Blue fluorescent amino acids as in vivo building blocks for proteins. | Chembiochem : a European journal of chemical biology 20100215 |
| Synthesis and antiproliferative activity of pyrrolo[3,2-b]pyridine derivatives against melanoma. | Bioorganic & medicinal chemistry letters 20100101 |
| Synthesis of 4- and 6-azaindoles via the Fischer reaction. | Organic letters 20091119 |
| Discovery of 4-azaindoles as novel inhibitors of c-Met kinase. | Bioorganic & medicinal chemistry letters 20090515 |
| Synthesis of C3-substituted 4-azaindoles: an easy access to 4-azamelatonin and protected 4-azatryptophan. | The Journal of organic chemistry 20080919 |
| Excited state tautomerization of azaindole. | Organic & biomolecular chemistry 20051021 |
| Preparation of 4-azaindole and 7-azaindole dimers with a bisalkoxyalkyl spacer in order to preferentially target melatonin MT1 receptors over melatonin MT2 receptors. | European journal of medicinal chemistry 20040601 |
| Unexpected ring transformation to pyrrolo[3.2-b]pyridine derivatives. Fused azolium salts. 22. | The Journal of organic chemistry 20030711 |
| Novel potent 5-HT(1F) receptor agonists: structure-activity studies of a series of substituted N-[3-(1-methyl-4-piperidinyl)-1H-pyrrolo[3,2-b]pyridin-5-yl]amides. | Journal of medicinal chemistry 20030703 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.